CRISPR Crops: The Application of Genome Editing on Agricultural Biotechnology
Ezekiel Bajomo • 2024-06-10
The novel development of genetic editing has had large impacts not just on research and disease, but on the food that is laid on our plates. 𝙄𝙣𝙨𝙩𝙞𝙩𝙪𝙩𝙚 - 𝘽𝙞𝙤𝙡𝙤𝙜𝙮 𝙃𝙖𝙧𝙫𝙖𝙧𝙙 𝙐𝙣𝙞𝙫𝙚𝙧𝙨𝙞𝙩𝙮
Precision DNA editing tools such as CRISPR allow us to fast-track years of selective breeding to create healthier, more sustainable, and tastier food for our increasingly food-insecure, and climate-challenged planet.
History of Selective Breeding
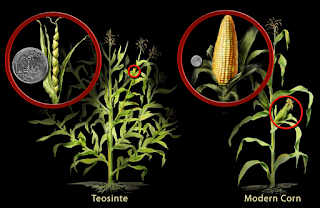
Throughout history, humans have always strived for improvement and innovation, seeking the best versions of everything – especially in agriculture. In ancient times long before the advent of genetic engineering, farmers would selectively breed agriculture with desired traits with hopes of increasing the frequency of those traits within a population. One of the earliest examples of selective breeding can be pinpointed to over 9,000 years ago when farmers in Mesoamerica (modern-day Mexico) started selectively breeding wild grass containing kernels called teosinte plants. Generations of breeding teosinte plants that possessed usually high volumes of kernels gave way to what we know today as Corn – one of the most important and influential crops in our modern world. Furthermore, some vegetables such as Broccoli were a human invention sprung from a process of selective breeding and cultivation; the vegetable was bred from the wild cabbage plant Brassica Oleracea in the Mediterranean region during ancient Roman times around the sixth century BCE. Although selective breeding became common practice early in human history, the understanding of the pertaining scientific processes did not come until Gregor Mendel and Charles Darwin.
Figure I. A side by side of the wild grass Teosinte and Modern Corn.
(Image credit: Nicolle Rager Fuller/National Science Foundation)
Gregor Mendel
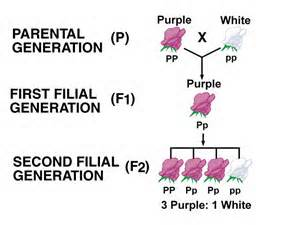
Gregor Mendel, also known as the father of genetics, was an Austrian-Czech monk whose pea plant experiments in 1857 helped discover the basic principles of heredity and increased the general understanding of inheritance – the study of how traits are genetically passed down in living things. He studied how certain traits, such as flower color, plant height, and seed shape, were passed down from one generation to the next. With his observations, he identified two types of traits: “dominant traits” that appeared in the offspring after a hybrid crossing, and “recessive traits”, which were hidden by the dominant traits, but would reappear in later generations.
Mendel discovered that when a plant inherits two identical alleles, versions of a gene, for a trait (homozygous), it will display that trait, whether it is dominant or recessive. However, if a plant has two different alleles (heterozygous), the dominant allele will always be expressed. This allowed me to come to two conclusions that are known as Mendel’s Laws of Heredity. Mendel’s Law of Segregation states that alleles separate randomly into gametes (sex cells), meaning that gametes carry one allele for a gene that is selected randomly. The Law of Independent Assortment states that genes for one trait are not inherited with genes of another trait, meaning that the inheritance of one trait does not interfere with another.
These principles help lay the groundwork for the understanding of genetics. It has also led to the discovery of non-mendelian genetics with principles that break the “rules” such as codominance and incomplete dominance. Nevertheless, Mendel’s foundational genetic concepts from his seminal experiments help in today’s society with the prediction and manipulation of genetic traits.
Figure II. Graphic illustrating Mendelian genetic principles with “P” denoting the dominant trait for the color purple, and “p” denoting the recessive trait for the color white. The parental generation represents a cross between a homozygous dominant and homozygous recessive plant. This results in the first filial generation that is heterozygous and displays the dominant purple trait. A self-cross in the F1 generation results in the F2 generation with a phenotypic ratio of 3 purple to 1 white. This example demonstrates Mendel’s genetic principles about dominant traits, recessive traits, homozygous heterozygous, and the law of segregation. Image Credit: (F1 Generation | Definition & Examples - Lesson | Study.com, n.d.)
Charles Darwin
Charles Darwin also helped expand the scientific understanding of selective breeding from his insights into natural selection and evolution. His groundbreaking work “On the Origin of Species” introduced the concept of natural selection – a mechanism in which organisms suited for their environment tend to survive and reproduce. Through extensive research and personal observations of the finch bird species on the Galápagos islands, Darwin documented the process of evolution in species over time through small, inheritable changes. He noted that these small changes could accumulate over time, leading to the adaptation of a certain environment or even the creation of a new species.
The concept of natural selection is founded upon the foundations of competition, variation, and heritability within nature. Natural selection differs from artificial selection in terms that one is propagated through environmental pressures and the other by humans, but both selectively pick and reward the organisms that suit their needs the best.
Darwin and Mendel together both brought scientific understanding, research, and a manifold of data to an ancient practice. Their ideas provide a foundation that scientists continue to build upon today.
Biotechnology Breakthrough
For most of history, causing favorable genetic mutations within an organism could only occur with the breeding of organisms with favorable traits. This process was hindered by natural genetic variations in populations and required long periods of time for significant changes. However, a biotechnology breakthrough in 1972 completely changed the field. In that year, Stanford biochemist Paul Berg successfully inserted DNA from a bacterium into the DNA of a monkey-infecting virus called SV40, creating the first DNA molecule made from the genetic code of different organisms. This achievement resulted in what is known today as hybrid DNA or recombinant DNA.
Recombinant DNA technology led to the birth of modern biotechnology and immediately had wide-reaching applications across various fields. In the pharmaceutical industry, recombinant bacteria were engineered to create human insulin, enabling industrial-scale and consistent production of the critical medication for diabetics. Soon after, the applications of recombinant DNA technology extended beyond bacteria, In 1980, a lab led by Thomas E. Wagner successfully transferred a rabbit gene into a mouse, creating the first transgenic mammal.
GMOs
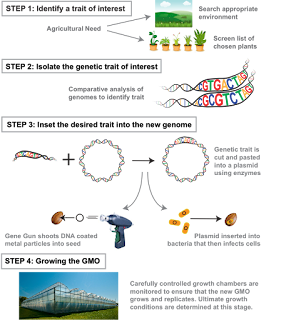
Continued breakthroughs eventually led to DNA recombination in plants or what is commonly referred to as Genetically modified organisms (GMOs). Plants or animals that have been created with genetic engineering are considered GMOs. The process of creating a GMO is fairly easy. Scientists begin by finding a desirable trait that they would like to replicate within nature. These traits could include increased survivability within a specific environment or unusually high nutritional content. Often, these traits are found by studying plants that live in extreme environments and screening the nutritional values of thousands of plants. Once the specific trait is recognized, scientists then proceed to isolate the specific gene responsible for the trait within the organism. Techniques such as “Seed chipping”, a rapid and accurate system of DNA extraction that allows for the kernel’s genome to be studied, help scientists close in on their gene of interest. After isolating the desired gene, scientists proceed to insert it into a different genome. This process is often done by using restriction enzymes to cut open a bacteria’s plasmid, inserting the DNA fragment in it, and joining it together with DNA ligase. This bacteria is then introduced to the seed. Lastly, the growth of genetically modified organisms is heavily monitored and placed in special growth chambers to ensure safe and healthy growth. Common bioengineered products are corn, soybeans, and potatoes.
Figure III. A summarization of the steps of creating a GMO. A trait of interest is identified, isolated, and inserted into a genome of interest. Lastly, the growth of the GMO is monitored. Image Credit: (SITNFlash, 2015)
CRISPR
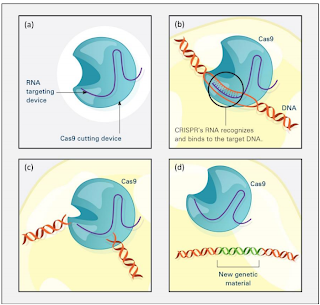
The development of the Nobel-prize winning gene-editing tool CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, has revolutionized the agriculture industry. The CRISPR-Cas9 complex is a quick and highly efficient molecule that allows for precise double-stranded cuts in DNA to happen, allowing for edits, insertions, and deletions within the genome. It is also extremely easy to engineer CRISPR-Cas9 molecules since all they require is a guide RNA to direct them to a specific location. This provides a strong advantage over other genetic editing tools such as Zinc finger nucleases and TALENs (Transcriptor Activatior-Like Effector Nucleases) and other DNA recombination techniques because full proteins have to be engineered for each new task; it would be akin to “building a new phone for each new app that you want to use” as said by biochemist Feng Zhang. CRISPR’s intuitive design also makes it a superior alternative to mutagens – mutation-causing agents such as certain chemicals and radioactive substances used to induce random mutations in plants in hopes of finding a desirable one.
Figure IV. A graphic of how the CRISPR Cas9 complex functions. Credit: NIGMS/NIH
CRISPR’s precise gene-editing capabilities have significantly enhanced the potential and efficiency of introducing and creating new, desirable traits in plants. Plants edited using CRISPR-Cas9 are not considered GMOs because the technique doesn’t add foreign DNA from different organisms; instead, it accelerates the process of selective breeding using the plant’s own genes. This revolutionary technology can positively influence various aspects of culture from taste to disease resistance.
Taste and Innovation
Unfortunately for our taste buds, some of the healthiest foods for our bodies have the most repugnant tastes, but with CRISPR this can be changed. Pairwise, a food and agriculture startup company that focuses on creating innovative products with CRISPR, decided to hone its attention on mustard greens. Mustard greens possess extremely high levels of nutritional content such as vitamins C and K, which makes them healthier than other popular greens such as lettuce (Karlson et al., 2022); although, mustard greens and other vegetables in the Brassicaceae family suffer from bitter tastes that disincentivize people from taking them.
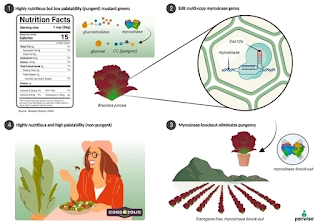
Researchers in Pairwise’s team identified the pungency in mustard greens to sulfur-containing compounds called isothiocyanates, which is derived by hydrolyzation of glucosinolates by the enzyme myrosinase. Isothiocyanate's pungent nature can be attributed to its functional group composed of nitrogen, carbon, and sulfur that stimulates the sensory receptors in the mouth similar to chili peppers. Through genomic sequencing and bioinformatics analysis, the location of the genes responsible for creating the myrosinase enzyme was then found, and a guide RNA was engineered to match the sequences of the gene’s coding region. The CRISPR Cas9 complex is then delivered to the mustard green cells, usually through direct DNA transfer techniques, directed by the guide RNA to create a double-stranded break at the myrosinase gene target site. The cell’s natural repair of mechanisms of the gene through non-homologous end joining fixes the break but effectively disrupts the gene’s function in the process. Polymerase chain reactions (PCR) and sequencing are used to confirm whether the cell went through the desired effect, and after it is validated, the cells are cultivated into plants with heavy monitorization during the process.
Figure V. The process of making mustard greens more palatable. The cause of the pungent taste is identified in the myrosinase enzyme, the gene creating myrosinase is targeted and loses its function using editing from CRISPR-Cas9, myrosinase can no longer catalyze the hydrolyzation of sulfur-containing chemical compounds, and mustard greens lose their bitter taste, which incentivizes people to eat the highly nutritious food. (Karlson et al., 2022)
In May 2023, Pairwise launched their purple and green mustard greens salad blend product called Purple Power Baby Greens Blend, making it the first CRISPR-edited food product sold in the United States. Their product is also even available in select restaurants across several states. Pairwise scientists have also recently developed the world’s first seedless blackberry and have plans to create the first pitless cherry. A meaningful portion of consumers avoid blackberries due to their seeds, so this innovation could greatly enhance the accessibility of these fruits. Additionally, the removal of thorns from these fruits helps reduce food waste and improve efficiency and economics for the farmers. With their ingenuity and innovative products, Pairwise’s goal of doubling the consumption of fresh fruits and vegetables nationwide is very plausible. Incentivizing people to eat tasty healthy foods of their own volition can be a strong tactic against fighting the obesity pandemic affecting 42% of adults in the U.S. (Overweight &Amp; Obesity Statistics, 2024).
Climate Resistance
One of the most pressing and challenging problems of our age is climate change: man-made shifts in global weather and climate patterns caused by the burning of fossil fuels and the release of greenhouse gases into the atmosphere. The effects of climate change range from warmer temperatures to extreme droughts, intense wildfires, and disastrous storms. These climate consequences will adversely impact agriculture and farming by weakening growing conditions, destroying environmental health, and making crops vulnerable to infection.
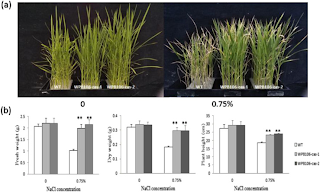
Rice, an important food crop, is at risk, and its destruction can turn global food security into disarray for the 60% of the world population that relies on it, especially in communities in Africa and Asia (Chauhan et al., 2017). A large threat to rice is abiotic stress – negative stress caused by inorganic factors – through salinity and drought from climate change. Increased salinity, or excessive salts in the soil, decreases a plant's ability to uptake water and therefore grow. Researchers have used CRISPR-Cas9 to knock out the OsRR22 gene in rice, which codes for an amino acid response regulator transcription factor, which increases salt tolerance when removed (Takakgi et al., 2015). When the edited rice plants were evaluated against a control group, the edited plants did significantly better.
Figure VI. The evaluation of a control rice plant, WT, and two CRISPR-Cas9 edited homozygous mutant rice plant lines, WPB106-cas-1 and WPB106-cas-2. While all plants possess similar values for weight and height in freshwater with 0 NaCl concentration, there is a statistically significant difference between the weight and height of the CRISPR-edited plants and the wildtype in water with a 0.75% NaCl concentration (Zhang et al., 2019).
Results from the experiment prove that CRISPR-Cas9 editing can be crucial in preserving important crops in the wake of droughts and extremely dry conditions. In a similar study, researchers have also improved rice plants' survivability in drought conditions using targeted gene knockout with CRISPR-Cas9. An important feature of plants is their stomata, which are pores that regulate water loss through opening and closing. Researchers have used CRISPR-Cas9 to knock out an early stomata developmental gene EPFL9 in rice, resulting in times smaller stomata density on the leaf surface of rice plants (Yin et al., 2017). A decrease in stomata density leads to less water loss, allowing plants to retain more water in drought conditions.
Disease Resistance
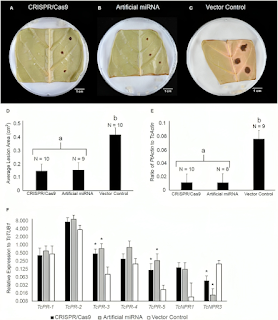
Chocolate, a beloved sweet around the world, faces many disease-related risks that could upend global production. The fundamental ingredient of chocolate is cacao beans which grow from cacao trees, Theobroma cacao, that are largely cultivated in West Africa. The backbone of the worldwide chocolate industry and many cacao-exporting countries rely on cacao trees, but a study from the College of Agricultural Sciences at Penn State finds that severe fungal outbreaks destroy 20-30% of cacao pods annually before harvest. However, CRISPR-Cas9 has proved to be a perfect solution for the cacao’s woes. Researchers identified a TcNPR3 gene in cacao that suppresses a defense response and hypothesized that removing the gene or reducing its function would increase resistance to disease (Fister et al., 2018). This hypothesis was tested by introducing the cacao pathogen Phytophthora tropicalis to a control group cacao leaf, a CRISPR-Cas9 edited leaf without the TcNPR3 gene, and a leaf with artificial microRNA that reduced the expression of the TcNPR3 gene. The visible signs of infections were then evaluated.
Figure VII. Image depicting visible signs of infection on each respective leaf. Graph D shows the average lesion area of the infected leaves; there is a statistically significant difference between groups a and b. Graph E shows the average ratio of pathogen actin (protein) to cacao actin, and again there is statistical significance between group a and b. Graph F shows the relative expression of other defense-related genes after the pathogen infection. (Fister et al., 2018) Data from the research shows that knocking out the TcNPR3 gene in cacao allows them to fight infections much more efficiently, as shown by smaller lesion sizes and less expressed protein. This provides a large step for creating biotechnology products to deal with the severity of pathogenic outbreaks in cacao plants, especially as they mutate and worsen over time.
Stigma of Gene Editing in Food and Agriculture
The current applications of CRISPR-Cas9 in agriculture have barely touched the surface, as the potential within the field is almost limitless; however, public perception and the current stigma against genetically modified products are large drawbacks that hold back a wide acceptance of the mechanism. For decades, a “GMO” label on food has created a stigma on products that has created a fear of them around. Oftentimes, GMO products are accused of causing cancer, being poisonous to ingest, and being a bane to the environment, despite the overwhelming scientific evidence that proves the opposite. Even though CRISPR-Cas9 edited products lack the “GMO” label, they will inevitably receive similar persecution by the wider public simply because DNA is still altered. The sanctity of DNA that people in the public hold can be attributed to what Scientific American writer Stefaan Blancke describes as “psychological essentialism.” The phenomenon “makes us think of DNA as an organism’s “essence” - an unobservable and immutable core that causes the organism’s behavior and development and determines its identity.” Therefore, when this “immutable core” is edited, people immediately call for foul play and denigrate the resulting products as unnaturally occurring and “Frankenfood” – a reference to the 1818 novel Frankenstein written by Mary Shelley telling the story of a scientist named Victor Frankenstein who deals with the irreversible consequences of deciding to “play God” by artificially creating a creature from old body parts and chemicals. Although, the “frankenfood” argument faces a few problems as many common foods we eat today are not naturally occurring. Broccoli, Kale, Brussels Sprouts, and Cauliflower are all non-naturally occurring plants that are only present today because of years of genetic modification through selective breeding of the parent plant Brassica oleracea, yet I doubt anyone has ever seen these “genetically modified organisms” ever get attacked before. Modern biotechnology allows us to perform many processes of the past in much more efficient, safer, and healthier ways, but it's up to us to fight the persistent stigma and fear around the products.
Conclusion
The development of CRISPR-Cas9 gene editing technology has significantly transformed the field of agriculture, offering revolutionary precision and efficiency in modifying plant genomes. This tool has upended other techniques such as selective breeding and refined genetic modification techniques in GMOs. However, CRISPR is not without its challenges. Some important considerations include difficulties in delivering gene-editing components to target cells, potential off-target effects where unintended parts of the genome may be altered, and concerns about potential loss of biodiversity within crops. Despite these potential risks and perceived drawbacks, CRISPR technology will undoubtedly continue to be a tool used to address climate change and food insecurity as long as they persist. Continued innovations and refinements will be crucial in overcoming the aforementioned challenges and fully harnessing the biotechnological capabilities to create truly sustainable, safe, and efficient worldwide agricultural systems.
References:
40 Things to Know: The world’s first transgenic mammal was developed here | Ohio University. (n.d.). https://www.ohio.edu/medicine/news-center/blog/40-things-know-worlds-first-transgenic-mammal-was-developed-here-ohio-university
Ahmad, A., Jamil, A., & Munawar, N. (2023). GMOs or non-GMOs? The CRISPR conundrum. Frontiers in Plant Science, 14. https://doi.org/10.3389/fpls.2023.1232938
Bartels, M. (2024, February 20). Tweaking vegetables’ genes could make them Tastier--And you’ll get to try them soon. Scientific American. https://www.scientificamerican.com/article/tweaking-vegetables-genes-could-make-them-tastier-and-youll-get-to-try-them-soon/
Blancke, S. (2024, February 20). Why people oppose GMOs even though science says they are safe. Scientific American. https://www.scientificamerican.com/article/why-people-oppose-gmos-even-though-science-says-they-are-safe/
Chauhan, B. S., Jabran, K., and Mahajan, G.. (2017). Rice Production Worldwide. Cham: Springer International Publishing. https://scholar.google.com/scholar_lookup?author=B.+S.+Chauhan&author=K.+Jabran&author=G.+Mahajan+&publication_year=2017&title=Rice+Production+Worldwide
Definition of mutagen - NCI Dictionary of Cancer Terms. (n.d.). Cancer.gov. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mutagen
F1 Generation | Definition & Examples - Lesson | Study.com. (n.d.). study.com. https://study.com/academy/lesson/f1-generation-definition-offspring-quiz.html
Fister, A. S., Landherr, L., Maximova, S. N., & Guiltinan, M. J. (2018). Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma cacao. Frontiers in Plant Science, 9. https://doi.org/10.3389/fpls.2018.00268
GalapagosIslands.com. (n.d.). Charles Darwin - The Origin of Species | Galapagos Islands. https://www.galapagosislands.com/info/history/charles-darwin.html
Gene Editing – Digital Media kit. (2020, November 5). National Institutes of Health (NIH). https://www.nih.gov/news-events/gene-editing-digital-press-kit
How are GMOs made? (2019, March 2). Sciencing. https://sciencing.com/gmos-made-6453138.html
Insight, F. (2020, August 27). INFOGRAPHIC: How did genetic modification get us to kale? Food Insight. https://foodinsight.org/infographic-how-did-genetic-modification-get-us-to-kale/
Karlson, D., Mojica, J. P., Poorten, T. J., Lawit, S. J., Jali, S., Chauhan, R. D., Pham, G. M., Marri, P., Guffy, S. L., Fear, J. M., Ochsenfeld, C. A., Chapman, T. A., Casamali, B., Venegas, J. P., Kim, H. J., Call, A., Sublett, W. L., Mathew, L. G., Shariff, A., . . . Rapp, R. (2022). Targeted Mutagenesis of the Multicopy Myrosinase Gene Family in Allotetraploid Brassica juncea Reduces Pungency in Fresh Leaves across Environments. Plants, 11(19), 2494. https://doi.org/10.3390/plants11192494
Limeri, L. (2023, January 11). Chapter 2: Artificial selection. Pressbooks. https://raider.pressbooks.pub/biology2/chapter/2-artificial-selection/
Nutrition, C. F. F. S. a. A. (2024, March 5). GMO crops, animal food, and beyond. U.S. Food And Drug Administration. https://www.fda.gov/food/agricultural-biotechnology/gmo-crops-animal-food-and-beyond
Overweight & Obesity Statistics. (2024, June 27). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity
Pairwise develops first seedless BlackBerry with transformative CRISPR technology. (2024, August 1). Pairwise. https://www.pairwise.com/news/pairwise-develops-first-seedless-blackberry
Sgn. (2022, January 28). Is broccoli Man-Made? | Gardening in Orange County New York. https://blogs.cornell.edu/master-gardeners-cce-oc/2022/01/28/is-broccoli-man-made/
SITNFlash. (2015, August 11). How to make a GMO - Science in the news. Science in the News. https://sitn.hms.harvard.edu/flash/2015/how-to-make-a-gmo/
The Nobel Prize in Chemistry 1980. (n.d.). NobelPrize.org. https://www.nobelprize.org/prizes/chemistry/1980/berg/facts/
United Nations. (n.d.). What is climate change? | United Nations. https://www.un.org/en/climatechange/what-is-climate-change
Walker, K. (2022, March 28). The future of food? CRISPR-Edited agriculture. Food and Drug Law Institute (FDLI). https://www.fdli.org/2021/11/the-future-of-food-crispr-edited-agriculture/
Wenham, L. (2024, June 26). CRISPR in agriculture: Applications, benefits & risks. Automata. https://automata.tech/blog/crispr-agriculture/
Yin, X., Biswal, A. K., Dionora, J., Perdigon, K. M., Balahadia, C. P., Mazumdar, S., Chater, C., Lin, H., Coe, R. A., Kretzschmar, T., Gray, J. E., Quick, P. W., & Bandyopadhyay, A. (2017). CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Reports, 36(5), 745–757. https://doi.org/10.1007/s00299-017-2118-z
Zhang, A., Liu, Y., Wang, F., Li, T., Chen, Z., Kong, D., Bi, J., Zhang, F., Luo, X., Wang, J., Tang, J., Yu, X., Liu, G., & Luo, L. (2019). Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Molecular Breeding, 39(3). https://doi.org/10.1007/s11032-019-0954-y
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.