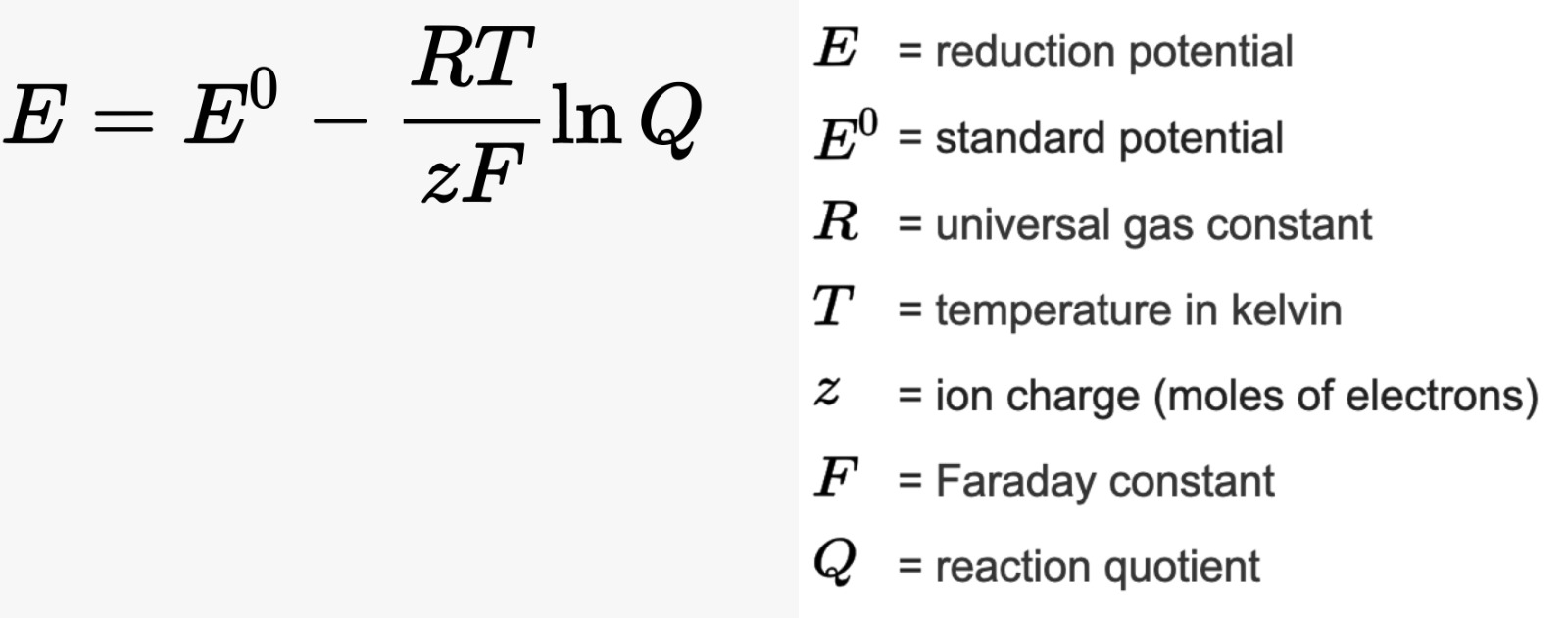Transition Metals and it's properties
Rishikesh Maharaj • 2024-06-26
𝙄𝙣𝙨𝙩𝙞𝙩𝙪𝙩𝙚 - 𝙄𝙡𝙛𝙤𝙧𝙙 𝘾𝙤𝙪𝙣𝙩𝙮 𝙃𝙞𝙜𝙝 𝙎𝙘𝙝𝙤𝙤𝙡 Transition metals, occupying the central block of the periodic table, show a unique set of chemical properties that separate them from other elements. These properties are largely attributed to their partially filled d-orbitals, which enable a wide range of oxidation states, complex formation, and varied reactivity.
Transition Metals
and their chemical properties Transition metals, occupying the central block of the periodic table, show a unique set of chemical properties that separate them from other elements. These properties are largely attributed to their partially filled d-orbitals, which enable a wide range of oxidation states, complex formation, and varied reactivity. Transition metals include widely known elements such as iron, copper, and gold, each playing critical roles in numerous chemical processes and applications. The main feature of transition metals is their ability to form stable and diverse compounds. This versatility arises from their electronic configuration, particularly the involvement of d-electrons in bonding and the resulting flexible valence states. This allows transition metals to participate in a multitude of chemical reactions, form coloured compounds, and allow for magnetic properties. Their unique behaviour is significant not only in industrial processes but also in biological systems and material science. Understanding the chemical properties of transition metals involves exploring their electron configuration, periodic trends, bonding capabilities, coordination chemistry, redox behaviour, reactivity, and the influence of these factors on their electronic spectra and magnetism. This article delves into these aspects, explaining the properties of transition metals and their chemical behaviour. Electron Configuration: The electronic configuration of transition metals is a fundamental aspect that defines their unique chemical behaviours. Transition metals typically have an electronic configuration that ends in (n – 1)d1 – 10ns0 – 2, such as iron (Fe), which has the electron configuration [Ar] 3d6 4s2. A distinctive feature of transition metals is their partially filled d-orbitals. These d-orbitals allow transition metals to exhibit a variety of oxidation states and form complex ions. This versatility in forming different positive ions, such as Fe2+ and Fe3+ in iron or Cu+ and Cu2+ in copper, allows transition metals to engage in numerous chemical reactions. The involvement of d-orbitals in bonding also enables the formation of complex ions with various geometries, contributing to their versatile chemistry – it is not usually expected to see metals outside of the d-block form huge coordination complexes, let alone bonds with organic ligands (e.g., haemoglobin, derived from iron). This characteristic makes transition metals crucial in many industrial processes and chemical reactions, acting as catalysts and intermediates.
Hafnium, a transition metal whose fifth shell is incomplete (10 electrons instead of 18) showing the tendency of seeing partially filled d-orbitals amongst transition elements. Periodicity:
The periodicity of transition metals further enhances their unique properties. As you move from left to right across a period in the d-block, the d-orbitals fill progressively, leading to subtle yet significant changes in atomic size, ionisation energy, and electronegativity. Transition metals generally have smaller atomic radii compared to s-block elements due to the increasing nuclear charge that pulls electrons closer to the nucleus. However, electron-electron repulsions in the d-orbitals can offset this effect slightly. Transition metals can form complex compounds with various ligands, a capability partly due to the availability of vacant d-orbitals. This leads to a diverse and rich chemistry, where transition metals exhibit multiple oxidation states and complex formation abilities. The presence of unpaired d-electrons also results in the formation of colourful compounds and magnetic properties. For instance, many transition metal compounds are brightly coloured because d-d electron transitions absorb specific wavelengths of light, and elements like iron display magnetic properties. These features demonstrate the importance of transition metals in both theoretical and applied chemistry. Chemical Bonding:
The chemical bonding in transition metals is uniquely defined by their ability to form diverse and complex bonds due to their flexible valence electron configurations and the availability of d-electrons. One of the distinctive aspects is their capacity for metallic bonding, characterised by the delocalisation of d-electrons, which enhances conductivity, malleability, and ductility. Additionally, transition metals can undergo extensive orbital hybridisation, combining their s, p, and d orbitals to create hybrid orbitals that facilitate strong covalent bonding through effective orbital overlap with other atoms. This ability to hybridise leads to the formation of robust and stable bonds, contributing to the creation of complex molecular geometries. The variable oxidation states of transition metals allow them to form a variety of covalent interactions, enabling them to participate in numerous redox reactions and catalytic processes. A well-known example of how these hybrid orbitals can lead to the formation of covalent bonds is HAuCl4 (Chloroauric Acid), or H2PtCl6 (Chloroplatinic Acid). Furthermore, transition metals exhibit ligand field effects, where the spatial arrangement and interaction of ligands with the metal centre cause the splitting of energy levels in the d-electrons. This results in unique electronic structures that are responsible for the vivid colours and magnetic properties observed in many transition metal complexes. The dynamic nature of their bonding, influenced by changes in oxidation states and the ability to form coordinate covalent bonds with a variety of ligands, underscores the versatile and intricate chemical behaviour of transition metals, making them indispensable in many areas of chemistry.
Left to right: H2PdCl4, H2PtCl6, HAuCl4
Coordination Chemistry:
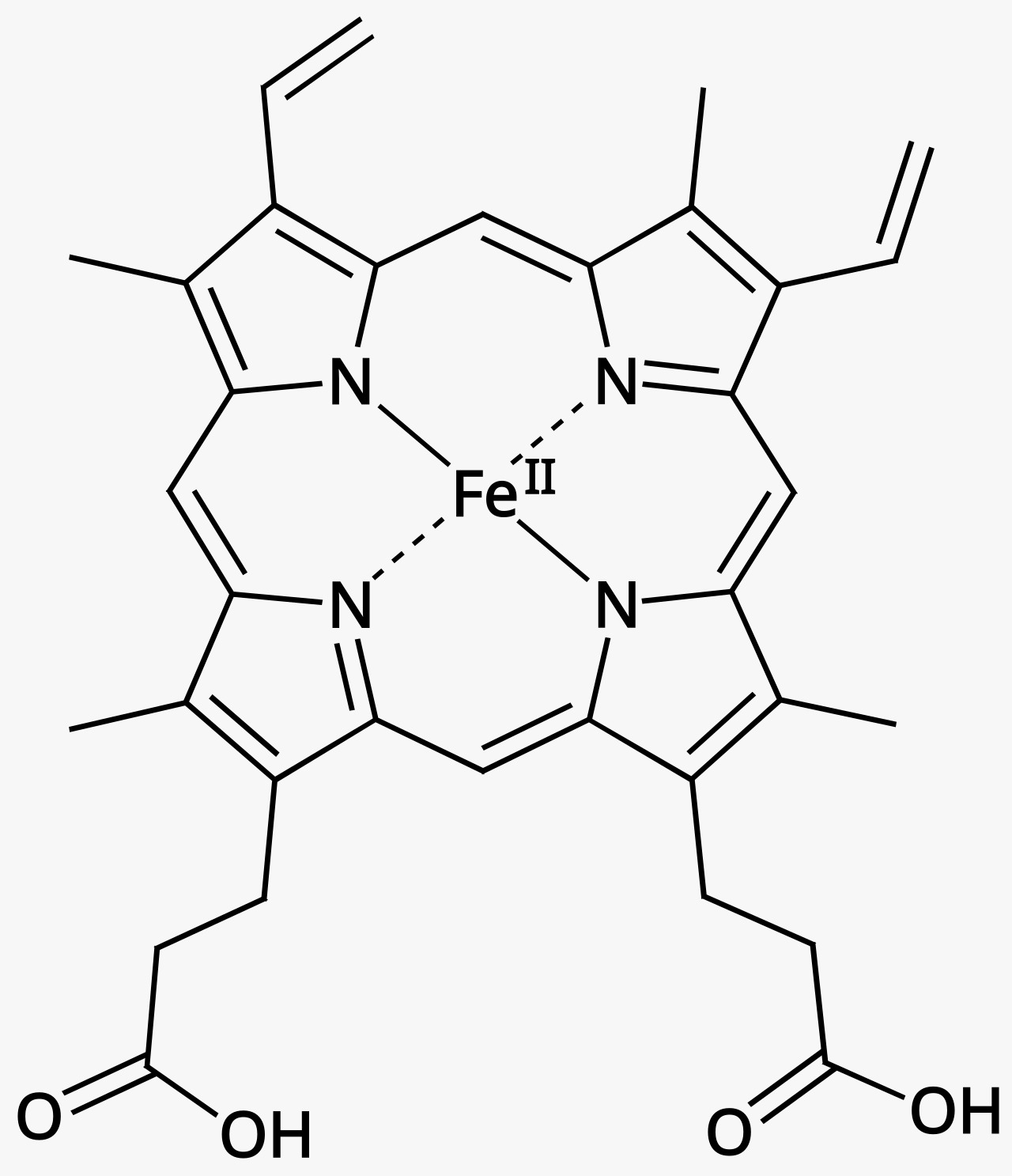
Coordination chemistry in transition metals arises from their ability to form stable complexes with a wide variety of ligands due to their unique electronic configurations and the presence of vacant d-orbitals. These metals can accommodate multiple ligands, creating complex geometries such as octahedral, tetrahedral, and square planar structures. The interaction between the metal ions and the ligands involves the donation of electron pairs from the ligands to the empty d-orbitals of the metal, forming coordinate covalent bonds. This bond formation is influenced by factors such as the size of the metal ion, its oxidation state, and the nature of the ligands. The flexibility in oxidation states allows transition metals to form complexes with different charge states, enhancing their reactivity and the range of possible chemical reactions. The crystal field theory explains the splitting of d-orbital energies in the presence of ligands, leading to characteristic absorption of light and resulting in the vivid colours often observed in transition metal complexes – seen in haemoglobin, which is responsible for the red colour of the blood. Additionally, the magnetic properties of these complexes are determined by the arrangement of electrons in the split d-orbitals, contributing to their varied and interesting magnetic behaviours. This ability to form stable, colourful, and reactive complexes makes coordination chemistry a pivotal aspect of transition metal chemistry, driving much of their application in catalysis, material science, and bioinorganic chemistry.
Heme b group (part of haemoglobin)
Oxidation-Reduction properties:
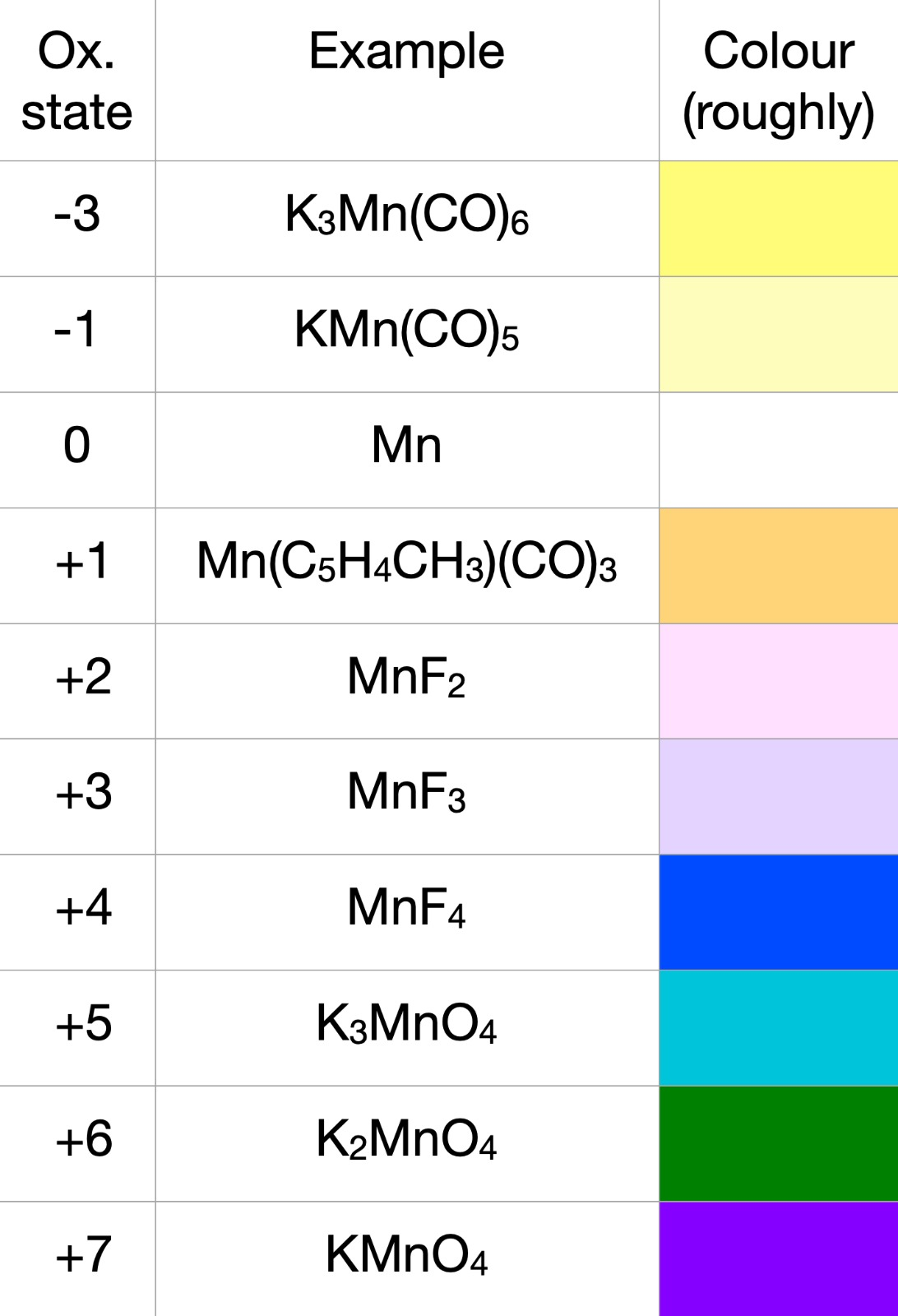
Redox properties are a significant chemical behaviour of transition metals, deeply intertwined with their electronic configurations and the variety of oxidation states they can adopt. These metals can readily undergo oxidation-reduction (redox) reactions due to their ability to lose and gain electrons, shifting between different oxidation states. For example, manganese exhibits a wide range of oxidation states from +2 to +7 (rarely going down to –3), as seen in compounds like MnO (manganese monoxide) and Mn2O7 (manganese heptoxide). Transition metals thus often act as catalysts in redox reactions, facilitating electron transfer by providing intermediate oxidation states. The redox behaviour is also significantly influenced by the ligand environment; for instance, the coordination of different ligands can stabilise particular oxidation states and alter redox potentials. In aqueous solutions, the standard reduction potentials of transition metals are vital for predicting the spontaneity of redox reactions. The Nernst equation (shown below) is instrumental in calculating the effective reduction potential under varying conditions, emphasising the environmental impact on redox behaviour. Furthermore, the interaction with ligands can lead to the formation of complex redox-active species, where the metal centre cycles through various oxidation states during the reaction process. A classic example is the catalytic cycle of the enzyme cytochrome c oxidase in cellular respiration, where iron and copper centres undergo redox changes to facilitate electron transfer and energy production. These dynamic redox properties enable transition metals to play pivotal roles in energy conversion, environmental remediation, and synthetic chemistry, underscoring their versatility and indispensability in both natural and industrial processes.Redox properties are a significant chemical behaviour of transition metals, deeply intertwined with their electronic configurations and the variety of oxidation states they can adopt. These metals can readily undergo oxidation-reduction (redox) reactions due to their ability to lose and gain electrons, shifting between different oxidation states. For example, manganese exhibits a wide range of oxidation states from +2 to +7 (rarely going down to –3), as seen in compounds like MnO (manganese monoxide) and Mn2O7 (manganese heptoxide). Transition metals thus often act as catalysts in redox reactions, facilitating electron transfer by providing intermediate oxidation states. The redox behaviour is also significantly influenced by the ligand environment; for instance, the coordination of different ligands can stabilise particular oxidation states and alter redox potentials. In aqueous solutions, the standard reduction potentials of transition metals are vital for predicting the spontaneity of redox reactions. The Nernst equation (shown below) is instrumental in calculating the effective reduction potential under varying conditions, emphasising the environmental impact on redox behaviour. Furthermore, the interaction with ligands can lead to the formation of complex redox-active species, where the metal centre cycles through various oxidation states during the reaction process. A classic example is the catalytic cycle of the enzyme cytochrome c oxidase in cellular respiration, where iron and copper centres undergo redox changes to facilitate electron transfer and energy production. These dynamic redox properties enable transition metals to play pivotal roles in energy conversion, environmental remediation, and synthetic chemistry, underscoring their versatility and indispensability in both natural and industrial processes.
Reactivity and Kinetics:
The reactivity and kinetics of transition metals are intricately linked to their electronic configurations, characterised by partially filled d-orbitals and multiple oxidation states, allowing them to participate in diverse redox reactions. Transition metals can undergo rapid ligand exchange through associative and dissociative mechanisms, with the former involving the formation of a transient higher-coordination intermediate, as seen in [Ru(NH3)6]3+ (Hexaammineruthenium ion), and the latter involving the initial loss of a ligand, creating a coordinatively unsaturated species, typical in [PtCl4]2– (Tetrachloroplatinate ion). The ability to form transient intermediates, such as π-complexes, facilitates catalytic cycles by stabilising intermediates and lowering activation energies. Transition metals also exhibit variable coordination geometries, enhancing their reactivity by providing diverse pathways for chemical transformations. Their unpaired d-electrons contribute to unique magnetic properties and influence electronic transitions, resulting in characteristic colour changes during reactions. An example of this is the complex [Cu(NH3)4]2+. Copper(II) has unpaired d-electrons, which contribute to its paramagnetic properties. When ammonia ligands coordinate with the copper ion, they cause a splitting of the d-orbital energies, leading to electronic transitions that absorb specific wavelengths of light. This results in the characteristic deep blue colour of the complex, illustrating how the electronic structure and ligand interactions influence both the magnetic properties and the visible colour of transition metal compounds. These properties, along with the dynamic nature of their ligand interactions and their capacity to form stable yet reactive intermediates, make transition metals essential in catalysis and advanced chemical processes.
Electronic Spectra and Magnetism
The electronic spectra and magnetism of transition metals are intrinsically linked to their unique electronic configurations and the presence of partially filled d-orbitals, which facilitate a range of electronic transitions and magnetic behaviours. The vibrant colours observed in many transition metal complexes are due to d-d transitions, where electrons absorb specific wavelengths of light to move from lower-energy to higher-energy d-orbitals. This process is described by crystal field theory, which explains how d-orbitals split into different energy levels in the presence of a ligand field, with the pattern of splitting depending on the geometry of the complex—octahedral, tetrahedral, or square planar—resulting in distinct absorption spectra. For example, the deep blue colour of the [Cu(NH3)4]2+ complex arises from such d-d transitions influenced by the ammonia ligands. Magnetism in transition metals is determined by the presence of unpaired d-electrons, generating magnetic moments. Paramagnetism, seen in species like Fe2+ and Mn2+, occurs when unpaired electrons align with an external magnetic field, enhancing its effect. Ferromagnetism, observed in metals such as iron, cobalt, and nickel, results from the parallel alignment of spins of unpaired electrons in adjacent atoms, creating a strong, permanent magnetic moment due to exchange interactions favouring parallel alignment. Antiferromagnetism, present in materials like MnO, involves adjacent atoms with unpaired electrons of opposite spins, cancelling out the magnetic moments and resulting in no net macroscopic magnetism. These properties lead to complex phenomena like magneto-optical effects, where the magnetic state of a material influences its optical properties. The relationship between electronic transitions and magnetic behaviours in transition metals is due to their flexible electronic structures, allowing various interactions with external fields and ligands.
In conclusion, the chemical properties of transition metals are defined by their unique electronic configurations, particularly the presence of partially filled d-orbitals. These properties enable a diverse range of oxidation states, complex ion formation, and varied reactivity, resulting in the formation of coloured compounds and distinctive magnetic behaviours. The ability to form stable and diverse compounds through complex coordination chemistry underscores their significance in numerous chemical processes, from industrial catalysis to biological functions. By exploring the underlying principles of electron configuration, periodic trends, bonding, redox properties, and their resultant electronic spectra and magnetism, we gain a comprehensive understanding of why transition metals behave the way they do. This knowledge highlights their roles and capabilities in both theoretical chemistry and practical applications, reminding us of their vast importance in scientific and industrial advancements.
References
Transition Metal: https://en.wikipedia.org/wiki/Transition_metal
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.