How do different organic compounds affect human health?
Pranav Haller • 2024-05-31
𝙄𝙣𝙨𝙩𝙞𝙩𝙪𝙩𝙚 - 𝙌𝙪𝙚𝙚𝙣 𝙀𝙡𝙞𝙯𝙖𝙗𝙚𝙩𝙝'𝙨 𝙎𝙘𝙝𝙤𝙤𝙡 Organic molecules are the most common chemicals used in our world and are found everywhere. From clothes to cars, they are essential to us as the building blocks of our world. However, despite having many applications, many of them often have adverse health effects on humans that are widely unknown. In this research project, I will delve deeper into the adverse health effects of organic molecules that we use on a day-to-day basis.
Introduction
Organic molecules are the most common chemicals used in our world and are found everywhere. From clothes to cars, they are essential to us as the building blocks of our world. However, despite having many applications, many of them often have adverse health effects on humans that are widely unknown. In this research project, I will delve deeper into the adverse health effects of organic molecules that we use on a day-to-day basis. I will discuss the most common organic molecules within each functional group. A functional group is a characteristic of an organic molecule that determines how it is named and its properties. Additionally, I will be discussing the side effects of the products formed when these molecules react as well as any health effects suffered from the production of these molecules.
Literature Review
Two sources that were immensely helpful to me were ‘Hydrocarbon Toxicity’ (14) and ‘Estimating acute cardiorespiratory effects of ambient volatile organic compounds’ (15). By combining information from both of these sources, I was able to get a comprehensive overview of the effects of organic molecules. While they did not have information on the effects of halogenoalkanes, I was able to get information for that from other sources. Both sources are published by PubMed Central which is a large library of all types of medical literature. Hence, I can conclude that both sources have a good reputation. Furthermore, I viewed the source from the National Library of Medicine which is an American governmental agency. This shows that both sources undoubtedly have a good reputation. Both sources have clearly referenced their sources, all of which are open source and most of which are other PubMed articles. Hence both sources can easily access the information they are writing about. Neither source has vested interest as they are both written by college and university professors who stand to gain nothing by lying in the article. Furthermore, the authors have expertise within this field as they teach at medical and environmental engineering colleges. Finally, the source seems neutral as it is simply reporting the findings of various medical studies done in the past. However, the second source has a lot of information that, even though it is concisely organised, is at a level above my understanding which made it quite difficult to interpret.
Effects of Alkanes
Alkanes are saturated hydrocarbons (a compound containing only carbon and hydrogen atoms) which means there are no double bonds between carbon atoms. They follow the general formula CnH2n+2. All alkanes are are extracted from crude oil in a process called fractional distillation. This is where the crude oil is heated up to a high temperature (350°-400°) and fed into a fractionating column where the temperature of the column decreases as it rises. Hence, shorter chain alkanes, which have less van der Waals forces, require less energy to boil, condense at the very top of the column whereas longer chain hydrocarbons, like Bitumen, are collected at the bottom of the column. This process is incredibly energy-consuming which means more fossil fuels have to burned to produce this which leads to higher carbon dioxide concentrations in the atmosphere.
Alkanes are most commonly used as fuels as they tend to completely combust meaning very little carbon monoxide (which is very poisonous) is produced. Alkanes in fuels tend to have a carbon chain length of 6-10 carbon atoms, most notably iso-octane (IUPAC name- 2,2,4-Trimethylpentane).
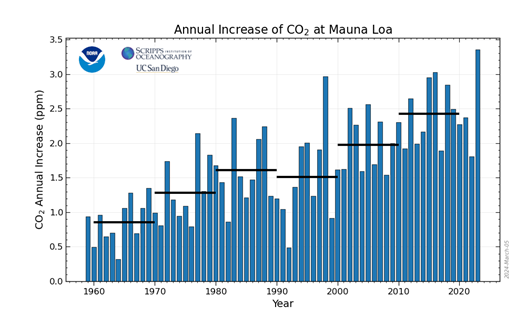
However, this means that more CO2 is released which leads to global warming. Furtherrmore, CO2 has a natural concentration of 0.04% in the air. However, as seen through this graph (1) detailing the increase of CO2 at the Mauna Lao laboratory in Hawaii (1) the rate of increase in CO2 has increased in recent years.
If this continues, the concentration of CO2 could increase to toxic levels. In fact, CO2 concentration of over 5% can lead to an increased respiratory rate and impaired concentrations while a CO2 concentration of over 10% leads to convulsions and could result in a coma or death (2). This is caused due to the Bohr effect. This is when CO2 dissolves in the blood, forming carbonic acid. This decrease in pH changes the tertiary structure of haemoglobin( the chemical responsible for carrying oxygen around your body) which causes it to decrease its affinity (attraction) for oxygen. This causes it to carry oxygen less effectively decreasing the amount of oxygen that is going around your body. If not enough oxygen reaches the brain, it can cause hypoxia(from the lack of oxygen) which leads to a coma or death.
However, a more likely way alkanes can harm you is through methane poisoning. Methane is the smallest alkane and the shortest hydrocarbon. It is very commonly found in cooking gas and in gas stoves in kitchens. While it has no side effects at low concentrations, at higher concentrations, the body cannot inhale enough oxygen and causes pneumonitis(lung inflammation)(3) and can result in a loss of consiousness. However, gas suppliers try to reduce the chance of this happening by making gas leaks more easy to spot. They do this by adding Mercaptin(methanethiol) into the gas supply which gives methane its distinctive rotten-egg odour(due to the presence of sulphur in the additive).
Overall, my research indicates that there are several ways in which alkanes can harm the human body, and as such, adequate care should be taken when handling them
Effects of Halogenoalkanes
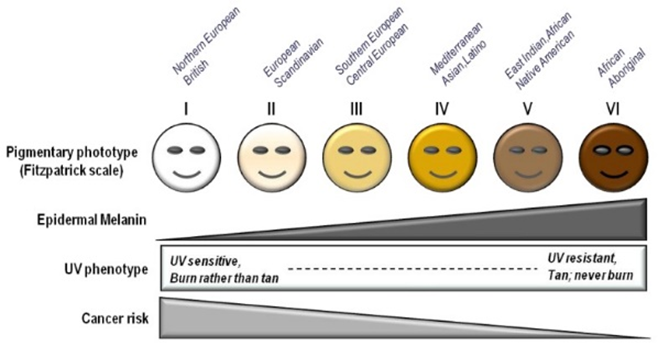
Halogenoalkanes are a functional group which has atleast one halogen group, most commonly flourine or chlorine. They have a wide variety of practical applications often used in refrigerants, solvents and in the pharmaceutical industry. Most halogenoalkanes do not occur naturally and are instead derived from organic precursors through a process called nucleophillic substitution. The most common type of halogenoalkanes are CFCs(Chlorofluorocarbons) which were used as refrigerants until 1978. Despite them being banned in 1978 by a global treaty, there is still a high concentration of them in the upper atmosphere. CFCs dissolve the ozone in the ozone layer which prevents dangerous UV radiation from the sun reaching humans. UV light is a mutagen meaning it causes more genetic mutations in cells to happen compared to a normal cell.However, when the UV light is filtered by the ozone, it makes it less harmful and is beneficial to humans by mediating the synthesis of Vitamin D(4) This leads to an increase in dermal and ocular conditions such as melanoma(skin cancer) and solar retinopathy(blindness caused by sun exposure)(5). The chart below shows how different demographics are likely to be affected by UV exposure(4).
Skin cancer is among the most common cancer types in the US. The below graph(6)
shows that, even though the death rate from melanoma is decreasing over time(due to advancements in cancer treatment technology), the rate of people getting melanoma every year is rising. While this may be due to a wide array of reasons(risk factors) such as your genetic makeup making you more susceptible to melanoma, the main factor is increased exposure to UV radiation. Hence, we can see that the increased use of CFCs in the 1970s has directly affected how prevalent skin cancer is modern society.
Overall, my research indicated that the use of CFC’s and their accumalation in the ozone layer has posed severe haelth risks to humans in the form of skin cancer by increasing the amount of UV radiation that can reach the earth’s surface.
Effects of Alkenes
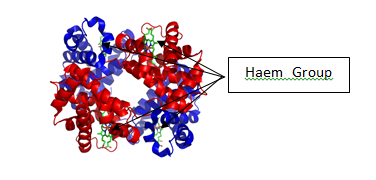
Alkenes are a functional group which are made of unsaturated hydrocarbons, meaning there is a double bond between adjacenet carbon atoms. This means that they undergo incomplete combustion when they are not in an excess of oxygen. This leads to the production of two extra side products. Carbon monoxide (CO) and pure carbon (C) in a solid state as soot. While alkenes are not used as fuels at massive scale, they are still used in some camping stoves. This poses several problems. Firstly, the production of carbon monoxide poses several health effects on humans. As mentioned earlier, haemoglobin is the chemical responsible for carrying oxygen around your body, by binding to it and forming oxyhaemoglobin. This occurs because of haemoglobin’s affinity for oxygen due to the presence of 4 haem groups(each containing a ferrous Fe2+ ion). The location of these ions can be seen in the diagram below (7).
However, haemoglobin has a stronger affinity for carbon monoxide than for oxygen by 210 times (8). This means that haemoglobin, in the presence of carbon monoxide, would bind to it rather than bind to the oxygen. This results in insufficient oxygen reaching the brain and vital organs. At concentrations of even 0.16%, death occurs within 8 hours; at 0.32% death occurs within 30 minutes (8). Secondly, the consumption of soot poses several health risks. Soot is widely considered as a carcinogen (9). This means it is a chemical that causes a person to get cancer. While soot is released from the incomplete combustion of alkenes it is also released from the combustion of coal in coal-fired power stations. An example of a city with a high soot concentration is Beijing where it mixes with the early morning moisture in the air to form Smokey fog or ‘smog.’ Soot also leads to inflammation of the airways, neurological disorders, and cardiovascular dysfunction (10).
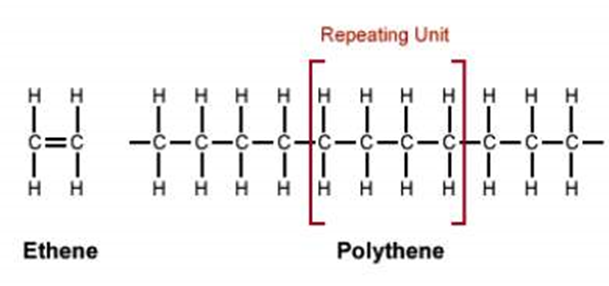
Alkenes are also used in the synthesis of plastics as they are the monomers and are combined in a series of condensation reactions (reactions which join two compounds and release a molecule of water) to form a polymer. The most common polymer is (poly)ethene or polyethylene. This is made of the simplest alkene, ethene(C2H4). The diagram (11) below shows how multiple smaller ethenes come together to form a longer polymer.
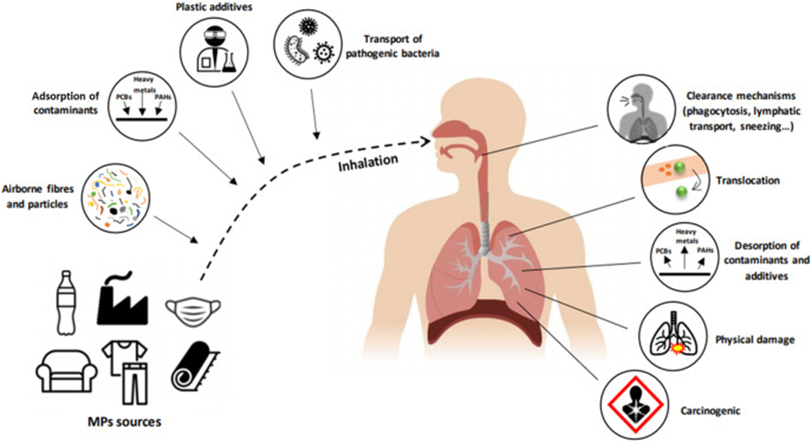
The polymer is used in a variety of products ranging from plastic bottles to cable insulation. Furthermore, it can be easily recycled and reshaped meaning it is a very versatile polymer. However, many people do not properly recycle these plastic products often leading them to accumulate in landfills or in the ocean. In the latter, they are broken down into very small pieces by erosion from ocean waves, called microplastics, and are very easily consumed by fish. When humans eat these fish, we indirectly consume the small, essentially invisible, plastic shards. While a small amount of microplastics will have a negligible impact, the bioaccumulation of microplastics causes progressively worsening conditions to occur. When ingested, microplastics pass through the gastrointestinal (GI) tract and has an abrasive effect on the walls of the intestines eventually leading to inflammation (12). Furthermore, microplastics change the pH in the gut affecting the gut microbiome and causing different, non-native, species of bacteria to grow in the gut. This leads to changed bowel habits. Additionally, if the microplastic has been tainted with heavy metals or polycyclic aromatic hydrocarbons (e.g. benzene; a carcinogen in itself), it can accumulate in the GI tract and can cause nausea, headaches, and abdominal pain. Microplastics also disrupt the natural secretion of hormones. This leads to various endocrine disorders, including metabolic disorders, developmental disorders, and even reproductive disorders (i.e., infertility, miscarriage, and congenital malformations) (12). Microplastics have also been recently shown to cause irreparable mitochondrial damage within cells. The mitochondria carry out aerobic respiration to release ATP (Adenosine Triphosphate) which is broken down(hydrolysed) to release energy and, if they are damaged, the cell will die as not enough ATP is produced leading to a lack of energy being released and thus the cell can’t carry out its basic functions and will die. While the damaged cell would be replaced with a functional cell, if the rate of cell death exceeds the rate of cell division and production, the body will be at severe harm. These are the effects of microplastics when they are ingested. However, the inhalation of microplastics also poses several health risks to humans. This diagram (13) outlines what happens when microplastics are inhaled.
While the human body has some defence mechanism to prevent airborne particulates (mucus, ciliated tracheal cells) from reaching the sensitive lung tissue, it is highly likely that some microplastics will reach the lungs regardless. Here they cause similar problems to those caused in the intestines. The microplastics are abrasive and so damage the delicate muscle in the lungs. This can trigger an immune response (triggered by the histamine released from the broken muscle cells) which would further increase inflammation in the lungs. Furthermore, if the microplastics are coated in heavy metals or polycyclic aromatic hydrocarbons, it will accumulate in the lungs exerting oxidative stress on the tracheal cells and will eventually cause tracheal cancer.
However, all of the conditions caused by the consumption of microplastics one way or another, occur over many years of plastic consumption. This is because plastic takes a long time to erode and form microplastics. Hence the concentration of microplastics in the sea and air is not high enough to cause these conditions instantly.
Overall, my research indicates that the combustion and consumption, in the form of microplastics, of alkenes poses severe health risks to humans when present at a high enough concentration.
Conclusion
To conclude, the main reason organic molecules have a negative impact is because they are incredibly different in their elemental makeup to the organic molecules in our body. Our body has organic molecules which contain oxygen, carbon and hydrogen as opposed to just carbon and hydrogen. Hence, when our immune system recognises it as foreign, it stimulates an immune response which causes more inflammation and worsens the effect of the organic molecule.
Additionally, the research I did produced similar results to the sources I covered in my literature review meaning that my conclusion is accepted by multiple notable sources.
While organic compounds have many different uses in our world, they also come with pretty considerable health effects. The different functional groups of organic compounds I covered in this project are just the most common ones. However, there are many more functional groups that I have not covered (aldehydes, ketones, carboxylic acids, esters etc.). However, the sheer number of health conditions caused by the use and production of just 5 chemicals that are incredibly common goes to show that, overall, most organic molecules have a severe negative effect on the functioning of the human body.
Bibiography
- US Department of Commerce, N. (n.d.). Global Monitoring Laboratory - Carbon Cycle Greenhouse Gases. [online] gml.noaa.gov. Available at: https://gml.noaa.gov/ccgg/trends/gr.html.
- Langford, N.J. (2005). Carbon Dioxide Poisoning. Toxicological Reviews, 24(4), pp.229–235. doi:https://doi.org/10.2165/00139709-200524040-00003.
- Jo, J.Y., Kwon, Y.S., Lee, J.W., Park, J.S., Rho, B.H. and Choi, W.-I. (2013). Acute Respiratory Distress Due to Methane Inhalation. Tuberculosis and Respiratory Diseases, [online] 74(3), pp.120–123. doi:https://doi.org/10.4046/trd.2013.74.3.120.
- D’Orazio, J., Jarrett, S., Amaro-Ortiz, A. and Scott, T. (2013). UV radiation and the skin. International Journal of Molecular Sciences, [online] 14(6), pp.12222–12248. doi:https://doi.org/10.3390/ijms140612222.
- Bruè, C., Mariotti, C., De Franco, E., Fisher, Y., Guidotti, J.M. and Giovannini, A. (2013). Solar Retinopathy: A Multimodal Analysis. Case Reports in Ophthalmological Medicine, 2013, pp.1–5. doi:https://doi.org/10.1155/2013/906920.
- (n.d.). Melanoma of the Skin - Cancer Stat Facts. [online] Available at: https://seer.cancer.gov/statfacts/html/melan.html#incidence-mortality [Accessed 28 Mar. 2024].
- By Zephyris at the English-language Wikipedia, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2300973
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.