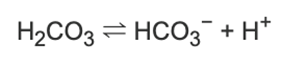The mechanism and importance of regulating lung ventilation
Lennon Crandle • 2024-06-28
𝗜𝗻𝘀𝘁𝗶𝘁𝘂𝘁𝗲 - 𝗠𝗲𝗱𝗶𝗰𝗶𝗻𝗲 𝗨𝗻𝗶𝘃𝗲𝗿𝘀𝗶𝘁𝘆 𝗼𝗳 𝗖𝗮𝗺𝗯𝗿𝗶𝗱𝗴𝗲 Ventilation is defined as the exchange of gases between the atmosphere and the bloodstream. During inspiration, oxygen enters the alveoli and diffuses into the bloodstream, whilst carbon dioxide diffuses from the arterial blood into the alveoli and is expelled in expiration. Both gases cross the alveolar epithelium and pulmonary capillary endothelium. The combined processes of inspiration and expiration is also what is termed as ventilation. In this artilce, I will discuss how lung ventilation is regulated by internal receptors and delve into the importance of this regulation, by illustrating the effects of a defective regulation.
Chemoreceptors
These are a type of receptor in the body that govern the principal mechanism of lung ventilation regulation, by responding to changes in blood pH. Two primary chemoreceptors exist, and these are central chemoreceptors and peripheral chemoreceptors. In principle, central chemoreceptors are chemo-sensitive areas, and they are found near cranial nerve roots VIII-XI, on the superior ventral surface of the medulla, close to the central pattern generator (CPG). Central chemoreceptors are surrounded by brain extracellular fluid (ECF) and cerebrospinal fluid (CSF). They play a dominant role in the regulation of the ventilatory response to hypercapnia.
Peripheral chemoreceptors, however, are found in carotid bodies at the bifurcation of the common carotid arteries, and in aortic bodies. These are above and below the arch of the aorta leaving the heart. The schematic for this is shown in figure 1. Peripheral chemoreceptors are functionally different to central chemoreceptors in that they are involved in the response to hypoxia, whilst central chemoreceptors are not. When the partial pressure of arterial oxygen (PaO2) drops to below 60 mmHg, peripheral chemoreceptors are able to detect this and increase firing of nerve impulses to the pneumotaxic centre. This explains why patients without carotid bodies have zero ventilatory drive in response to hypoxia. Central chemoreceptors however play a more functionally important role than peripheral chemoreceptors, which only make up 20-40% of response to hypercapnia.
Fig.1- Location of peripheral chemoreceptors
Stimulation of chemoreceptors
Central chemoreceptors are very sensitive to the partial pressure of arterial CO2 (PaCO2). An increase in PaCO2 means an increase in CO2 entering the CSF, since the blood-brain barrier is very permeable to CO2. CO2 then dissolves in solution to form carbonic acid, resulting in the following dissociation.
Therefore, an increase in CO2 in the CSF increases proton concentration, decreasing pH. Central chemoreceptors then detect this change, meaning that they are indirectly sensitive to PaCO2 and directly sensitive to changes in [H+]. This is what causes the stimulation of central chemoreceptors.
Peripheral chemoreceptors are stimulated by a similar mechanism. A decrease in PO2 with an increase in PCO2 results in the same decreased pH as mentioned. This stimulates dark, peptide-containing glomus cells in carotid bodies. These cells respond by inhibiting K+ ion channels, leading to increased depolarisation by Na+. This results in Ca2+ entry and the release of neurotransmitter, initiating an electrical impulse to be carried towards the CNS to augment alveolar ventilation
Both central and peripheral chemoreceptors use this mechanism to adjust alveolar ventilation in different external environments, for example, at high altitudes. In these conditions, atmospheric PO2 is lower and so the amount of oxygen inspired is also lower. This results in chemoreceptor-coordinated hyperventilation, causing a decrease in PaCO2 (it can drop from 40mmHg to 8mmHg). This allows an increase in PaO2 to levels compatible with life. At this stable level, hyperventilation is then inhibited, resulting in further decreases in PaCO2, making CSF in the brain more alkaline, as a decrease in arterial CO2 would mean a decrease in [H+]. This decreases the stimulation of central chemoreceptors and inhibits ventilation. This is a negative feedback-based mechanism of ventilation regulation. At this point, peripheral chemoreceptors take over. Central compensation is required to maintain ventilatory drive: over a few days, HCO3- gets removed from CSF and is excreted via the kidneys, resulting in a decrease in the potency of the carbonic acid/bicarbonate buffering system, decreasing the pH and allowing a continually higher ventilation rate. This is the proposed mechanism for the regulation of ventilation in hypoxic environments.
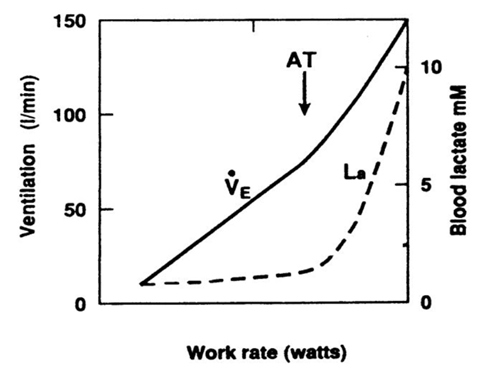
The regulatory mechanism used by chemoreceptors can also be a fail safe mechanism for the aforementioned buffer system. In the state of intense exercise, lactic acid is in excess due to respiration being predominantly anaerobic, and this exceeds the blood buffering power by bicarbonate. This has a net decrease on blood pH, which is detected by chemoreceptors and they respond accordingly. This results in an increase in ventilation for the same proportionate increase in exercise intensity – this is what is called the anaerobic threshold.
Fig.2 The anaerobic threshold (AT)
Other peripheral contributors to the regulation of ventilation
Pulmonary stretch receptors, proprioceptors, irritant receptors, baroreceptors, pain sensors and temperature sensors are all said to play a role in the regulation of lung ventilation. They do this each via various mechanisms, for example proprioceptors may be important under conditions requiring increased ventilation accompanied by forced expiration.
The role of breathing mechanics in the regulation of ventilation
It may be argued that the physical mechanics of breathing plays a partial role in regulating lung ventilation. Transmural pressures refer to the pressure difference across a wall, such as a tissue or organ. It is calculated by subtracting the pressure on the outside of the wall from that of the inside. Transpulmonary pressure (PL) refers to the difference in pressure between the inside of an alveoli (PA) and that in the pleural cavity (Ppl). Therefore PL = PA – Ppl. The pressure inside the alveoli at the start of inspiration is 1 atm, or is set at ‘0’ cmH2O. The basal intrapleural pressure (pressure in the pleural cavity, of the pleural fluid) is -5cmH2O. It changes from -5 to -8cmH2O on inspiration. This increases the transpulmonary pressure from 5 (0 - -5) to 8 (0 - -8) and so causes a net outwards flow of gas in the alveolus. This is what causes lung inflation and ventilation. To deflate, the pleural pressure must therefore increase and hence transpulmonary pressure must decrease. Therefore, changes in the transmural pressures in the lungs and the physical mechanics of breathing can be said to somewhat regulate lung ventilation, as without their modulation ventilation can neither increase nor decrease.
The importance of regulating lung ventilation
As outlined, both central and peripheral chemoreceptors work to regulate ventilation, increasing and decreasing PO2 where required. If this alveolar ventilation is not regulated, the supply of arterial oxygen does not meet aerobic demand, and this can lead to hypoxia. There are different types of hypoxia, characterised by their different natures. Hypoxic hypoxia is caused by a low percentage saturation of haemoglobin with oxygen. Anaemic hypoxia results from a reduction in the oxygen-carrying capacity of the blood (low iron, haem etc). Circulatory hypoxia is where too little oxygenated blood is delivered to tissue by blood vessels. Histotoxic hypoxia is caused when cells cannot use oxygen, such as when they have been poisoned by cyanide. Then there is hypercapnic hypoxia (asphyxia), wherein the PaCO2 is excessively high.
In the deregulation of lung ventilation, VA cannot be increased when required, so not enough oxygen is delivered to respiring tissues. This forces respiration to be conducted anaerobically, producing lactic acid – an excess of which can lead to muscle fatigue and cramps.
In general, in its deregulation, ventilation is adversely imbalanced with perfusion. The calculation that quantifies this ventilation-perfusion relationship is the ventilation-perfusion ratio (VA/Q). This calculation is important in assessing the ventilatory state of a patient. If alveolar ventilation and blood flow are not matched, this will be illustrated by this ratio. When there is inadequate ventilation, the ratio is low, and less gas exchange occurs across the affected alveoli. This results in a fall in capillary PO2 and a rise in related PCO2. Additionally, hypoxic vasoconstriction will cause the diversion of blood flow to better ventilated parts of the lung. However, this does not have a great effect due to the haemoglobin of these already better ventilated aspects of the lung already having a high percentage saturation with oxygen. This means that PO2 in the blood cannot be increased by a large margin, keeping arterial PCO2 higher and providing a stimulus for hyperventilation, detected by chemoreceptors as previously discussed.
In conclusion, the internal mechanism that governs the regulation of lung ventilation is based at the central and peripheral chemoreceptors. These respond directly to changes in pH brought about by differential PaCO2 levels and send electrical impulses to the brain accordingly. Chemoreceptors are important in maintaining steady ventilation when [H+] exceeds the potency of the CO2/HCO3- buffer system. Furthermore, the regulation of lung ventilation is important in maintaining the steady state of PaO2 and PaCO2 homeostasis to avoid hypoxia, maintain aerobic respiration, as well as maintaining adequate VA/Q ratios.
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.