Unveiling the Island of Stability and Superheavy Elements
Rafin Hossain and Hari Rathan • 2025-05-01
𝙄𝙣𝙨𝙞𝙩𝙪𝙩𝙚 - 𝙉𝙚𝙬𝙝𝙖𝙢 𝘾𝙤𝙡𝙡𝙚𝙜𝙞𝙖𝙩𝙚 6𝙩𝙝 𝙁𝙤𝙧𝙢 Nuclear stability is a cornerstone of atomic science, describing the behavior of atomic nuclei over time. A stable nucleus neither spontaneously emits radiation nor undergoes decay, while an unstable one will. The balance between protons and neutrons within the nucleus plays a pivotal role in determining its stability.
Most naturally occurring elements have stable isotopes, which exhibit ideal proton-to-neutron ratios. However, as elements become heavier, maintaining this delicate balance becomes increasingly challenging, leading to shorter half-lives and faster radioactive decay.
The concept of the “island of stability” offers a potential exception to this trend. While most superheavy elements - those with atomic numbers greater than 104 - are highly unstable, it is hypothesised that certain isotopes of these elements may possess extraordinary stability. These isotopes may have “magic numbers” of protons and neutrons, granting them unusually long half-lives compared to their unstable counterparts. The idea was first proposed in the mid-20th century, as physicists speculated that certain superheavy elements might behave differently, potentially forming a stable “island” within an otherwise chaotic nuclear landscape. As the atomic number increases beyond uranium (atomic number 92), naturally occurring elements become increasingly unstable, prompting scientists to question whether there is a fundamental limit to the number of protons a stable nucleus can hold.
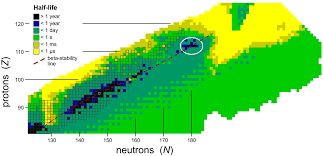
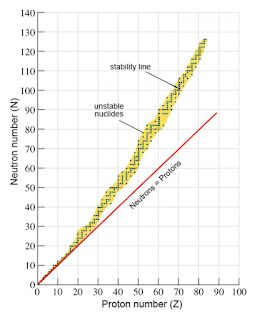
Figure 1 - Graph showing relationship between stability and proton/neutron number
Figure 1 also highlights this rise in instability, likely as a result of increased forces of repulsion between protons within the nucleus
Nuclear Shell Theory and Magic Numbers
The theory of nuclear shells, inspired by the behavior of electrons in atomic orbitals, provides the foundation for understanding the island of stability. “Magic numbers” (2, 8, 20, 28, 50, 82, 126) represent numbers of protons or neutrons that form complete nuclear shells, leading to greater stability. When protons or neutrons reach these magic numbers, their energy levels are filled, and the nucleus becomes more tightly bound, making it less likely to undergo decay.
The strong nuclear force, which binds protons and neutrons together, competes against the electromagnetic force that repels protons due to their positive charge. In heavier elements, the repulsive electromagnetic force becomes more pronounced, destabilizing the nucleus. However, within the island of stability, the configuration of protons and neutrons in closed shells can suppress this repulsion, leading to greater stability and longer half-lives.
Superheavy Elements and Quantum Mechanics
Quantum mechanics, particularly the Schrödinger equation, plays a crucial role in understanding nuclear stability. The Schrödinger equation describes the behavior of quantum particles, such as protons and neutrons in a nucleus, by determining their energy levels. The Hamiltonian operator, which represents the total energy of the system (kinetic and potential), is used to calculate the stability of nuclei by finding the energy levels of protons and neutrons.
The stability of superheavy elements is influenced by the interplay between the strong nuclear force and the electromagnetic repulsion between protons. In these elements, the arrangement of protons and neutrons in closed shells, as predicted by quantum mechanics, enhances the strong nuclear force, helping to overcome the destabilizing electromagnetic repulsion. This results in the potential for superheavy elements to exist for longer periods, forming part of the island of stability.
The Search for Superheavy Elements
International collaborations, such as those between the Joint Institute for Nuclear Research (JINR) in Russia and the Lawrence Livermore National Laboratory (LLNL) in the United States, have made significant strides in synthesizing superheavy elements. By using particle accelerators to bombard lighter elements like calcium with heavy target materials such as curium or plutonium, scientists aim to create superheavy nuclei. While elements like flerovium (114) and livermorium (116) have been discovered, their half-lives are still extremely short, indicating that the quest for the island of stability is ongoing.
The synthesis of superheavy elements is an incredibly challenging task. Fusion reactions, required to create these elements, involve colliding atomic nuclei with enough energy to overcome the repulsive forces between positively charged protons. Even when successful, the newly formed elements decay almost instantly, making their detection and study difficult. To detect and measure these elements, specialized instruments such as time-of-flight detectors and mass spectrometers are employed to analyze the decay patterns and energies of these isotopes.
The Potential of Superheavy Elements
The discovery of stable or long-lived isotopes from the island of stability could have profound implications across multiple scientific fields. In nuclear medicine, for example, stable superheavy isotopes could be used in diagnostic imaging and cancer treatment. Radioactive isotopes are already crucial in medical imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). However, the isotopes used today have limitations in terms of half-life, energy emission, and availability.
Superheavy isotopes with longer half-lives and more predictable decay patterns could revolutionize medical imaging by offering better tracers for monitoring biological processes over time. These isotopes could also be utilized in radiotherapy, where alpha-emitting isotopes, which have high energy but short ranges, could be used to target cancer cells more precisely, minimizing damage to surrounding healthy tissues. This would represent a significant advancement in personalized cancer treatments.
The Future of Superheavy Elements
Producing and detecting superheavy elements remains an expensive and resource-intensive endeavor. However, advancements in accelerator technology and more sensitive detectors are likely to improve the feasibility of synthesizing these elements. As researchers continue their pursuit, the eventual discovery of stable superheavy isotopes could lead to breakthroughs in nuclear physics, medicine, and materials science, marking a new era in scientific understanding.
In summary, the island of stability offers a fascinating frontier in nuclear physics, promising the discovery of superheavy elements with unprecedented stability. While their synthesis remains a formidable challenge, the potential benefits in fields like medicine, energy, and materials science make this pursuit worthwhile. The future of superheavy elements holds the promise of advancing human knowledge and improving lives across the globe.
References
Burbidge, E.M., Burbidge, G.R., Fowler, W.A. and Hoyle, F., 1957. Synthesis of the elements in stars. Reviews of Modern Physics
Clark, J.P., 2015. Superheavy Elements: The Quest for the Island of Stability. Physics Today
Eichler, R. et al., 2013. Chemical characterization of element 114, flerovium. Nature
Hamilton, J.H., Hofmann, S. and Oganessian, Y.T., 2013. Search for superheavy elements. Annual Review of Nuclear and Particle Science
Hofmann, S. and Münzenberg, G., 2000. The discovery of the heaviest elements. Reviews of Modern Physics, 72(3), pp.733-767
Oganessian, Y.T., 2007. Heaviest nuclei from 48Ca-induced reactions. Journal of Physics G: Nuclear and Particle Physics, 34(4),
Loveland, W., 2007. The production and decay of superheavy elements. Nature Reviews Physics, 2(1), pp.25-38. DOI: 10.1038/s41567-019-0697-1.
Sanderson, K., 2014. Superheavy element chemistry revealed. Nature, 505(7483),
Stoyer, M.A., 2006. Nuclear structure and reactions of superheavy elements. Nuclear Physics
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.