A Deep Dive into HeLa cells
Hari Rathan • 2024-07-11
Historically for many years, scientists attempted to create a cell line—a group of cultured cells that can be maintained outside the body. Achieving this difficult feat brought the potential to revolutionise science, enabling breakthroughs in medical research and the development of cures previously deemed impossible.
Despite these aspirations, all efforts were futile due to a lack of information on the required conditions for culture and the tendency of cells to die quickly. These limitations greatly hindered research. However, with advancements in medical knowledge, scientists eventually identified the basic conditions necessary for optimal cell culture: all cells require twelve amino acids to synthesize proteins, and glutamine is essential for metabolism. Additionally, maintaining a suitable pH of around 7± is crucial to avoid harmful effects. Despite this knowledge, providing all the nutrients in the correct proportions remains challenging, and even then, cells often still die. The most effective form of human cell culture currently comes from embryonic stem cells, though they do not offer the greatest depth of research. This paradigm shifted in 1951 when Dr. George Otto Gey observed something extraordinary. Henrietta Lacks, a 31-year-old Black tobacco farmer, visited Johns Hopkins Hospital for cervical cancer treatment, where a surgeon took a minor sample of her tumor. This sample would grow to become the first immortal human cell line, known as HeLa, contributing immensely to modern medicine. The cell’s name “HeLa” is derived from the first two letters of her first and last names.
Figure I. Cervical Carcinoma HeLa Cell Culture marked with Alexa Fluor Dyes. Olympus Life Science Dr. Gey's repeated attempts to create a cell line had previously failed. However, when he cultured Lacks’ tumor sample, he noticed that within a day, the cells had doubled and needed more space to grow. This process continued, and the cells never died as long as they were provided with correct space and nutrients. These "immortal cells" continue to be cultured today, and it is estimated that if all the HeLa cells ever grown were laid in a line, they would wrap around the Earth three times.
Immortalised cells aren’t immortal; they possess the ability to divide indefinitely surpassing a phenomenon called the Hayflick limit. Cells normally divide a set number of times based on the length of non-coding sections of DNA called telomeres
Figure II. DNA with yellow labeled Telomere caps. T.A Sciences
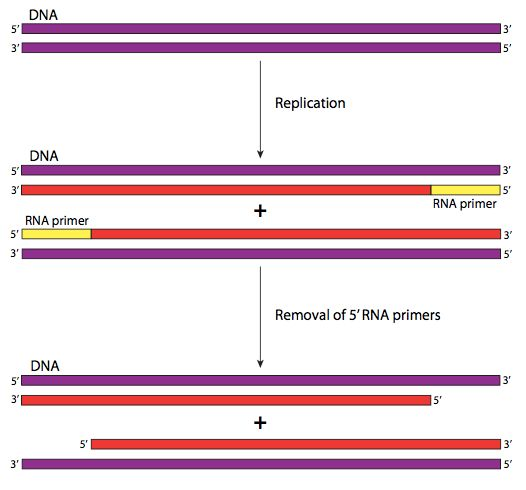
Every time a cell’s DNA is copied a small piece of the telomere is lost because of a phenomenon called the end replication problem
To copy our DNA our cells need an RNA primer. This primer tells DNA polymerase, the copy machinery of our cells, where to start. Each time the DNA is copied, a small portion of the telomere is lost, causing cells to eventually run out of telomeres and lose their ability to divide. Continuing to divide would start to cut into essential genes that control cell function. This limitation on cell division is one of the mechanisms that prevent normal cells from uncontrollably proliferating.
Figure III. Parts of the initiation and elongation phases of DNA replication - Part of the End-replication problem. This process occurs continuously in HeLa cells.
LibreTexts - Biology
Only certain cells in our bodies, like stem cells, can produce an enzyme called telomerase, which extends the length of telomeres allowing the cells to keep dividing. This process is normally tightly controlled, but in cancer cells, the telomerase gene can get turned back on allowing the cells to divide indefinitely. Similarly in HeLa cells, the telomerase gene is turned back on to allow the extension of telomeres.
Whilst ordinary cells would have died out, Lacks' cells were able to achieve this immortality due to a combination of factors: her uniquely aggressive cancer, the cells having multiple copies of the HPV-18 (human papillomavirus) genome which caused Henrietta Lacks’ cervical cancer by switching off her tumour suppressor gene, and having syphilis, which weakened her immune system, allowing the cancer to spread further. Her biopsy sample doubled every 20 to 24 hours. Dr. Gey created the HeLa cell line, without any consent, and made it available to researchers for free. These cells later became commercialized, although they were not patented, and Dr. Gey did not profit from them. Samples were sent globally, transforming treatment methods still in use today.
Another important aspect of HeLa cells is their widespread availability and ease of cultivation. Unlike many other types of human cells, HeLa cells can be easily grown in the laboratory and shared among researchers worldwide. This has allowed scientists to conduct a wide range of experiments and studies using HeLa cells, leading to numerous breakthroughs in various fields of research. Conversely, the unusual growth capabilities of the HeLa cell line led to the contamination of many cell cultures and ruined years of research, as discovered by Stanley Gartler in 1966.It was discovered that the HeLa cells could float on dust particles and could be transferred on unwashed hands and pipettes, and therefore due to cross contamination end up in other cell cultures. The cells were so prevalent , that just one could lead to the complete takeover of a culture.
Revolutionary Applications of HeLa Cells
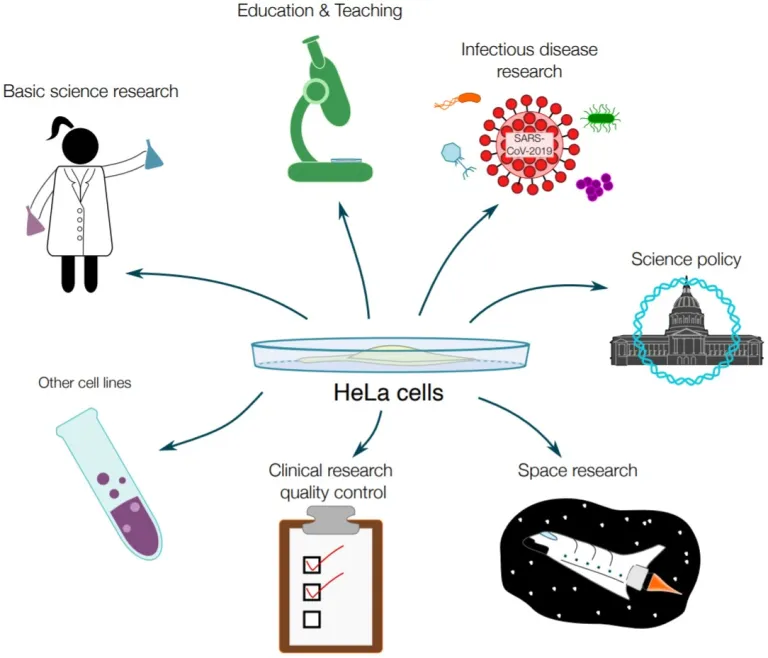
The HeLa cells weren’t just used for treatments. According to research conducted by Noel Jackson, a graduate student from Harvard University, HeLa Cells were dispatched into space during the Space Race, a 20th-century competition between Cold War rivals the United States and the Soviet Union, to investigate the repercussions of zero gravity on human cells. Furthermore, they were examined to evaluate the consequences of radiation exposure following nuclear bomb detonations. Moreover, the beauty industry employed HeLa cells to evaluate the potential side effects of new cosmetic products.
Figure IV. A graphic portraying a myriad of sectors that HeLa cells have been applied in. Not limited to, but including, space research, government policy, and teaching in secondary schools and universities across the world. Harvard GSAS Key Milestones in HeLa Cell Research
1953: The Polio Vaccine
During the development of the polio vaccine, scientists required a reliable cell line to test its efficacy. HeLa cells were found to be more susceptible and impossible to kill, unlike the costly monkey cells he used prior to poliovirus infection. Compared to other cell lines, HeLa cells were ideal for this purpose. The Tuskegee Institute, also the site of the infamous syphilis studies, was chosen to create a factory dedicated to manufacturing HeLa cells. Culturing of HeLa cells occurred at an unprecedented industrial scale, contributing to the rapid subsequent clinical trials and rollout of the vaccine. Since the implementation of the vaccine, two out of the three strains of the disease have been globally eradicated, and there has been a 99% decrease in wild polio cases, saving millions of lives.
Poliomyelitis, a motor neuron disease affecting the spinal cord, predominantly affected children in the 1940s and 50s, often leading to paralysis and reliance on devices like the 'iron lung' for breathing support. Transmission typically occurs through contact with contaminated faeces. This commonly transpires when children do not thoroughly wash their hands. Consumption of food or water contaminated with the virus is another route of transmission. Additionally, the virus can spread through airborne droplets when an infected child coughs or sneezes. Furthermore, the virus may persist in a child's faeces for several weeks. Children are most contagious immediately before and after they show signs of symptoms. The poliovirus is now on the verge of global eradication, marking a remarkable milestone in modern medical history. Central to this achievement was Jonas Salk, whose pioneering efforts led to the development of the first safe and effective polio vaccine. The World Health Organization (WHO) has highlighted that polio primarily affects children under five, with one in two hundred infections leading to irreversible paralysis. Among those paralyzed, 5-10% die when their breathing muscles become immobilized. HeLa cells played a crucial role in expediting the availability of the vaccine, as their high susceptibility to the virus and durability made them ideal for testing.
The HeLa cell facility at Tuskegee Institute allowed for successful vaccine testing by Salk. Over the past six decades, the vaccine has played a pivotal role in eradicating polio from the majority of countries worldwide. As of 2023, only a small number of individuals worldwide still depend on iron lungs for their survival, a testament to the vaccine's success. Paul Alexander and Martha Lilliard are among the few who still use iron lungs, highlighting the importance of vaccination and the impact polio has had on many lives.
1964: Treatment for Blood Disorders
Hydroxyurea and Sickle Cell Anemia
In 1964, HeLa cells were instrumental in studying the potential treatment benefits of the drug Hydroxyurea for blood disorders, particularly sickle cell anemia. Sickle cell anemia is a genetic disorder caused by a mutation in the hemoglobin gene, leading to the production of abnormal hemoglobin known as hemoglobin S. This mutation causes red blood cells to become rigid, sticky, and misshapen (sickle-shaped), leading to various complications such as pain, anemia, infection, and stroke.
Hydroxyurea was proved effective in cutting back on the frequency of painful crises and the need for blood transfusions in patients with sickle cell anemia. Studies using HeLa cells demonstrated that Hydroxyurea increases the production of fetal hemoglobin (HbF), which inhibits the sickling of red blood cells. By preventing the polymerization of hemoglobin S, Hydroxyurea helps maintain the red blood cells' normal shape, improving their function and lifespan. This discovery has led to Hydroxyurea becoming a cornerstone in the management of sickle cell anemia, significantly improving the quality of life for patients.
Leukemia and Cancer Research
HeLa cells have also been vital in leukemia research. Leukemia is a type of cancer that affects blood-forming tissues, including the bone marrow and the lymphatic system, resulting in the production of abnormal white blood cells. These abnormal cells crowd out normal blood cells, leading to symptoms such as anemia, infection, and bleeding.
Researchers have used HeLa cells to study the mechanisms of leukemia and to develop treatments targeting the disease. One significant contribution was in understanding how certain drugs can inhibit the proliferation of cancer cells. For example, studies on HeLa cells helped in the development of chemotherapy agents that target rapidly dividing cells, a hallmark of leukemia. By testing these agents on HeLa cells, scientists were able to evaluate their efficacy and toxicity, leading to the development of effective treatment protocols for leukemia patients.
Scientists have also used HeLa cells to identify important genes within Leukemia cells that allow them to evade apoptosis (programmed cell death). A novel Leukemia gene called MLAA-34 was found by researchers, but its function was originally unknown. By creating a HeLa cell line that expressed the MLAA-34 gene they were reliably able to test and observe its effects. They found that the expression of the MLAA-34 gene led to higher survival rates and lower rates of apoptosis when introduced to apoptosis-inducing chemical arsenic trioxide. Understanding the identity and function of genes such as these help scientists increase their knowledge of the disease as well as the ability to create highly tailored and specific treatments for leukemia.
Thalassemia and Genetic Research
Thalassemia is another genetic blood disorder that has benefited from research involving HeLa cells. Thalassemia is caused by mutations in the genes that encode for hemoglobin, resulting in reduced or absent production of one of the hemoglobin chains. This leads to ineffective erythropoiesis (production of red blood cells), anemia, and other complications.
HeLa cells have been used in genetic studies to understand the mutations responsible for thalassemia and to explore gene therapy approaches. By introducing specific genetic modifications into HeLa cells, researchers have been able to study the effects of these mutations and test potential gene editing techniques. These studies aim to correct the defective genes in patients with thalassemia, offering the potential for a cure rather than merely managing the symptoms.
Myelodysplastic Syndromes (MDS)
Myelodysplastic syndromes (MDS) are a group of disorders caused by poorly formed or dysfunctional blood cells due to abnormal development in the bone marrow. MDS can lead to severe anemia, infection, or bleeding, and may progress to acute myeloid leukemia (AML).
Research using HeLa cells has provided insights into the genetic and molecular mechanisms underlying MDS. By studying the genetic mutations and pathways involved in the abnormal development of blood cells, scientists have identified potential therapeutic targets. For instance, HeLa cells have been used to test new drugs that aim to modulate the signaling pathways implicated in MDS, leading to the development of targeted therapies that can improve outcomes for patients.
Hemophilia and Clotting Disorders
Hemophilia is a genetic disorder that causes the inability of blood to clot properly, leading to excessive bleeding. It is caused by mutations in the genes encoding clotting factors, such as factor VIII (hemophilia A) or factor IX (hemophilia B).
While HeLa cells are not directly used to produce clotting factors, they have been instrumental in understanding the genetic basis of hemophilia and in the development of gene therapy approaches. Researchers have used HeLa cells to study the expression and regulation of clotting factor genes, paving the way for the development of gene therapy techniques that aim to introduce functional copies of these genes into patients' cells. Clinical trials are currently underway to test the efficacy and safety of these gene therapy treatments, which have the potential to provide a long-term cure for hemophilia patients.
1983: HPV and Cervical Cancer
In the 1980s, virologist Harald Zur Hausen discovered that HeLa cells contained multiple copies of human papillomavirus 18 (HPV-18), which caused Henrietta Lacks’ cervical cancer by switching off her tumor suppressor gene. This particular strain is considered one of the most dangerous, and further research enabled the development of the HPV vaccine, now commonly administered to teenage girls. The vaccine has significantly reduced deaths from HPV by almost two-thirds.
HeLa cells have also been instrumental in understanding the mechanisms of cancer and developing treatments for various types of cancer. Zur Hausen’s groundbreaking research earned him the Nobel Prize in Physiology or Medicine in 2008 for demonstrating that viruses can cause certain types of cancer. This discovery was crucial in the development of HPV vaccines, credited with significantly reducing HPV infection rates among teenage girls.
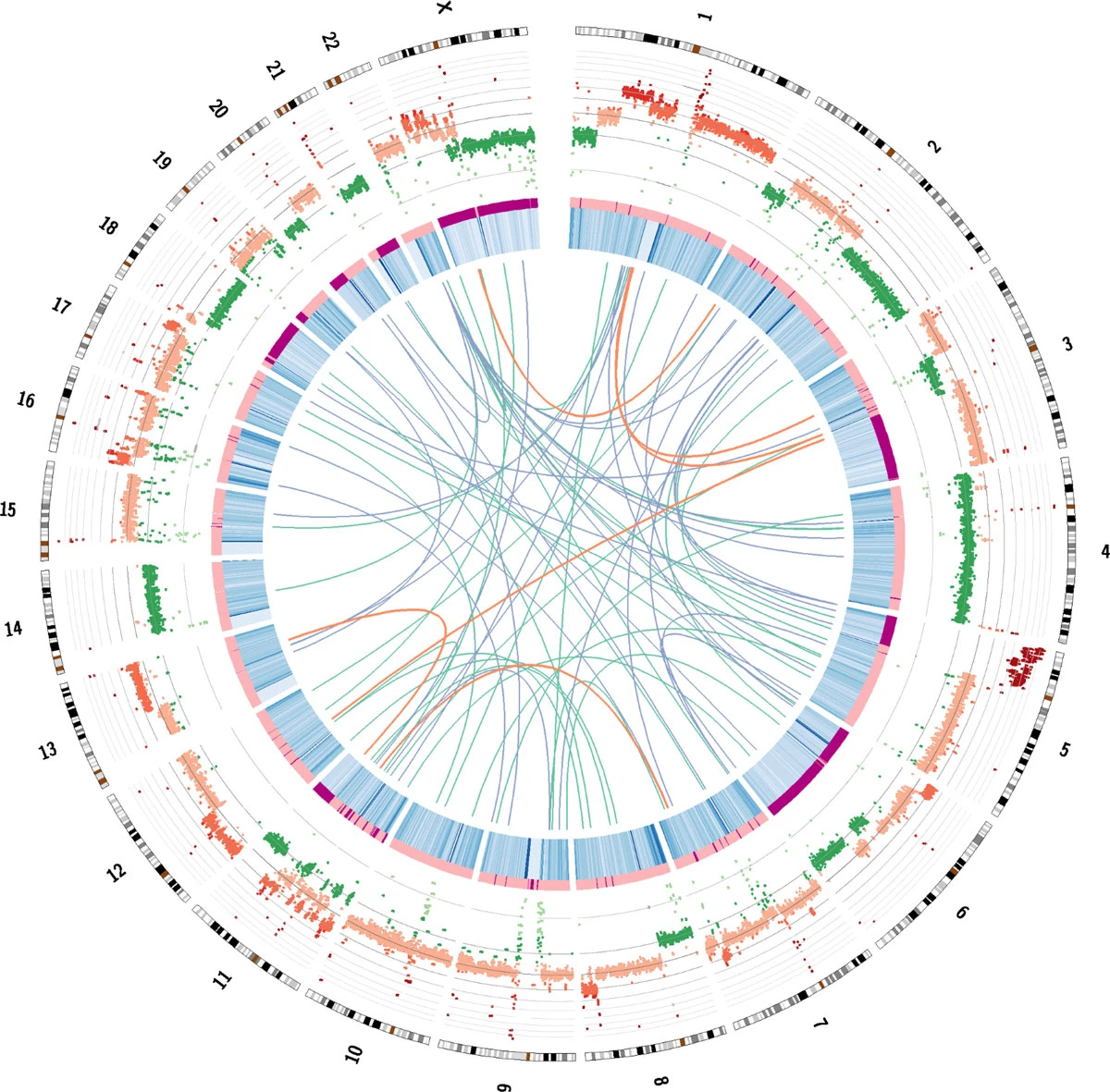
Figure V. A Circos plot demonstrating the genetic features of the HeLa cells. DNA and RNA sequencing were used to reveal and understand the genetic abnormalities with the nucleotides of the genome. Genome maps such as these created by scientists bring valuable information to understanding HPV and Cervical Cancer. Springer Nature
Mapping the Human Genome
HeLa cells have been extensively used to map the human genome, unraveling the intricacies of genetic code organization and function. They facilitated chromosome counting studies, aiding in understanding chromosomal abnormalities and genetic disorders. In the mid-1960s, John Watkins and Henry Harris created the first human-animal hybrids by fusing HeLa cells with mouse cells, paving the way for gene mapping techniques. These hybrid cells played a notable role in the early stages of gene mapping, contributing to the comprehensive mapping of the human genome during the Human Genome Project.
2020: Covid-19
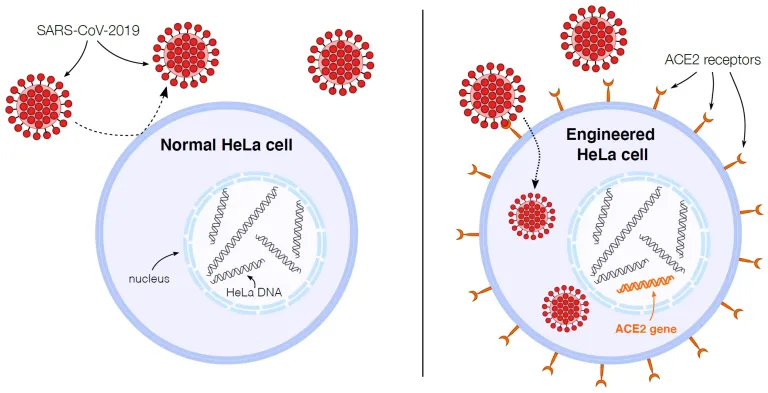
During the COVID-19 pandemic, HeLa cells were used to study the infectivity of the SARS-CoV-2 virus. It was found that this form of the virus was unable to infect HeLa cells as expected, prompting research into the cause. Scientists modified HeLa cells to produce and display the ACE2 molecule, enabling the virus to infect and replicate inside the cells. This research paved the way for the development of COVID-19 vaccines, estimated to have saved 20 million lives in one year.
Figure VI. As depicted in the graphic above, HeLa cells can be genetically engineered to display extremely high amounts of ACE2 receptors, allowing scientists to understand essential aspects of SARS-Cov-2 infectious ability through its viral spread. Harvard GSAS
- HIV Research
HeLa cells have been exposed to a range of viruses, including HIV, herpes, Zika, measles, and mumps, to enhance understanding of how to combat these pathogens. For instance, scientists discovered that a type of white blood cell known as a T cell has a surface protein called CD4, which HIV utilizes to infiltrate cells. By introducing CD4 to HeLa cells, they became susceptible to HIV infection, facilitating drug testing. More recently, researchers discovered that the Zika virus fails to replicate in HeLa cells, which could lead to new treatments or vaccines for Zika.
Ethical Controversies and Henrietta Lacks' Legacy
The story of Henrietta Lacks remained largely unknown until author Rebecca Skloot's bestseller "The Immortal Life of Henrietta Lacks" raised awareness about informed consent in scientific research. The whole issue illustrates the deep problem of racial inequality in the US at the time however when Lacks' tissue sample was taken, there was no legal or ethical requirement for doctors to obtain permission from the patient. Lacks had no knowledge of her cells being utilized in this manner as she died despite aggressive radiation therapy and surgery of her cancer in the same year 1951 October 4th at the age of 31. It was only 25 years after her death that her family learned about the cell line, which by then had contributed to numerous medical advancements.
Today, informed consent regulations are much stricter, but the ethical debate persists. If Henrietta Lacks had the choice and declined to donate her cells, many medical advancements might not exist. The balance between ethics and public benefit remains controversial, but greater awareness enables better decision-making.
References
- General Information and History
- HeLa Cells - Office of Science Policy
- Henrietta Lacks | Morehouse School of Medicine
- URL: Morehouse School of Medicine
- Reliability: High (reputable medical institution)
- HeLa - Wikipedia
- URL: Wikipedia
- Reliability: Medium (publicly editable, but usually well-sourced)
- The story of Henrietta Lacks and the uniqueness of HeLa cells
- URL: Morehouse School of Medicine
- Reliability: High (reputable medical institution)
- URL: Office of Science Policy
- Reliability: High (official government source)
- Impact on Science and Medicine
- Significant Research Advances Enabled by HeLa Cells
- URL: Office of Science Policy
- Reliability: High (official government source)
- 5 important ways Henrietta Lacks changed medical science
- Samuel, L. 2017. STAT
- URL: STAT
- Reliability: High (reputable science and medicine news source)
- Vessels for Collective Progress: the use of HeLa cells in COVID-19 research
- Jackson, N. 2020.
- URL: Science in the News
- Reliability: High (affiliated with Harvard Medical School)
- Significant Research Advances Enabled by HeLa Cells
- Ethical Considerations
- Frequently Asked Questions | Johns Hopkins Medicine
- URL: Johns Hopkins Medicine
- Reliability: High (prestigious medical institution)
- Henrietta Lacks: science must right a historical wrong
- Nature. 2020.
- URL: Nature
- Reliability: High (top-tier scientific journal)
- Immortal cells and informed consent: the legacy of Henrietta Lacks
- Kent, C. 2021.
- URL: Pharmaceutical Technology
- Reliability: High (industry-respected source)
- Frequently Asked Questions | Johns Hopkins Medicine
- Books and Literature
- Skloot R. The Immortal Life of Henrietta Lacks. New York: Crown Publishers; 2010.
- URL: Crown Publishers
- Reliability: High (well-researched and acclaimed book)
- www.sparknotes.com/lit/immortal life/plot analysis
- URL: SparkNotes
- Reliability: Medium (good for summaries, but not primary sources)
- Skloot R. The Immortal Life of Henrietta Lacks. New York: Crown Publishers; 2010.
- References on Polio and Sickle Cell Disease
- Polio
- Brazil Introduces Inactivated Polio Vaccine in National Immunization Program with Sanofi Pasteur Vaccine
- URL: European Pharmaceutical Review
- Reliability: High (trusted industry publication)
- Development of the Polio Vaccine: A Historical Perspective of Tuskegee University’s Role in Mass Production and Distribution of HeLa Cells
- Turner, T. Journal of Health Care for the Poor and Underserved. 2012;23(4a):5-10.
- URL: Project MUSE
- Reliability: High (peer-reviewed journal)
- Poliomyelitis (polio)
- URL: WHO
- Reliability: High (World Health Organization)
- The Virus Behind the Cancer
- URL: Yale Medicine
- Reliability: High (reputable medical school)
- Brazil Introduces Inactivated Polio Vaccine in National Immunization Program with Sanofi Pasteur Vaccine
- Sickle Cell Disease
- Sickle Cell Disease (SCD) Treatment & Management: Approach Considerations, Hydroxyurea Therapy, Transfusion
- URL: Medscape
- Reliability: High (reputable medical resource)
- [Internet]. Hematology.org
- URL: Hematology.org
- Reliability: High (American Society of Hematology)
- Sickle Cell Disease (SCD) Treatment & Management: Approach Considerations, Hydroxyurea Therapy, Transfusion
- References on COVID-19
- COVID-19 vaccines saved an estimated 20 million lives in 1 year
- URL: CIDRAP
- Reliability: High (Center for Infectious Disease Research and Policy)
- COVID-19 vaccines saved an estimated 20 million lives in 1 year
- General References
- George Otto Gey - Wikipedia
- URL: Wikipedia
- Reliability: Medium (publicly editable, but usually well-sourced)
- Space Race - Wikipedia
- URL: Wikipedia
- Reliability: Medium (publicly editable, but usually well-sourced)
- George Otto Gey - Wikipedia
- Academic and Technical References
- Culture Conditions and Types of Growth Media for Mammalian Cells
- Yang, Z., Xiong H. 2012.
- URL: IntechOpen
- Reliability: High (peer-reviewed open access book)
- Culture Conditions and Types of Growth Media for Mammalian Cells
- The references from reputable sources such as official government websites, respected medical institutions, peer-reviewed journals, and highly regarded news sources are considered highly reliable. Wikipedia is generally reliable but should be cross-verified due to its editable nature.
Financial and Ethical Considerations
The question of who should benefit financially from HeLa cells is also contentious. While pharmaceutical companies profited, the Lacks family remained in poverty. In 2013, the NIH included two members of the Lacks family in decisions about who can use HeLa cells. By doing so, we can continue to benefit from the invaluable contributions of HeLa cells while respecting the rights and dignity of individuals like Henrietta Lacks whilst efforts have been made to address these ethical concerns and honor Henrietta Lacks' legacy, highlighting the importance of considering ethical issues in scientific research and respecting the rights and autonomy of individuals whose cells are used, although informed consent has come a long way since the 1950s, we still face several challenges. Notably, in developing countries there have been cases of violation of consent, often with regard to misinforming patients of potential dangers associated with procedures and trials.
Just last year, a settlement was reached by the Lacks family and biotechnology company Thermo Fisher, which profited billions from the nonconsensually taken HeLa cells, even after the ethical issues of the origin of the cells started to arise. Although the matter between the two parties was resolved in closed-door negotiations, the situation demonstrates the further strides needed to be taken by companies and societies to prioritize the well-being, consent, and health of people over the allure of profits.
Unfortunately, Henrietta Lacks’ story is just one of many cases of racial exploitation in medicine and research. In 1932, the United States Public Health Service began the Tuskegee Study, where they initially enrolled 600 black men – 399 with syphilis and 201 without syphilis – so that they could understand the effects of syphilis in an untreated black person. Throughout the study, the men were treated in a sub-human way comparable to lab rats and were at times given mercury and arsenic, which are harmful to the human body. Even in 1947 when penicillin, one of the strongest treatments for syphilis, became available none of the men were treated and helped, but rather left to suffer and deal with the harrowing symptoms of their disease. It wasn't until the 1960s, after the discovery of Henrietta Lacks, that a whistleblower from inside the USPHS named Peter Buxtun brought complaints to the U.S. government. These complaints were ignored until Buxtun decided to leak documents from the study in 1972, making it fully available to the public. The study ended a few months after and a $10 million dollar settlement was negotiated outside of court. It is estimated that 100 men died in the study.
Rebecca Skloot and Peter Buxtun are extremely brave individuals who played pivotal roles in highlighting injustices when nobody else saw them. Their efforts raise an important question: would these injustices still have persisted if not for their interventions? It appears that companies, corporations, and governments often benefit from injustices only until they are publicly exposed and held accountable, rather than addressing issues on their own. Even with the advancements in science, issues like these increase the mistrust that people, especially minorities, have in their medical systems. In the recent COVID-19 pandemic, there was a large surplus of people who rejected the vaccine. Specifically, using the CDC COVID-19 vaccine tracker, researchers found that black individuals living within a 750-mile radius of Tuskegee were slower to get vaccinated than other white individuals in the same area, and other black individuals in other parts of the United States. Given the rough history of discrimination and inequality in medicine and research, it is understandable that many people lack trust and belief in the medical system of their country. Undoing the fatal mistakes of the past will be a long path.
Conclusion
The HeLa cell line has revolutionised modern medicine, aiding in the development of treatments and cures for various diseases. However, ethical concerns related to informed consent and the rights of individuals remain significant. Scientists and policymakers must address these issues to ensure responsible and ethical research. By doing so, we can continue to benefit from HeLa cells while respecting the dignity of individuals like Henrietta Lacks. As we look to the future, finding a balance between ethical tissue acquisition and the need for research materials is crucial.
References
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.