Discussing the hormonal regulation of male and female gametogenesis
Lennon Crandle • 2024-07-14
𝙄𝙣𝙨𝙩𝙞𝙩𝙪𝙩𝙚 - 𝙈𝙚𝙙𝙞𝙘𝙞𝙣𝙚 𝘾𝙖𝙢𝙗𝙧𝙞𝙙𝙜𝙚 𝙐𝙣𝙞𝙫𝙚𝙧𝙨𝙞𝙩𝙮
Abstract
30 million sperm cells are produced per day in the adult male, as opposed to 1-2 oocytes each month in the female. Sperm gets released in testicular fluid whilst oocytes are released in follicular fluid. In the male, there is a large number of renewable stem cells that remain at puberty, versus the few oocytes left at puberty in females, with no stem cells. There is a continuous output of two main hormones in the man: androgens and inhibin, whilst in females there is sequenced release of two main steroid hormones: rising oestrogens, leading to oocyte release, leading to rising progesterone. Inhibin output rises pre-oocyte release and remains high after it. In the male testicular activity and gamete generation is continuous, in contrast with the female where ovarian activity and gametogenesis is cyclic/discontinuous.
This essay will discuss the hormonal regulation of both male and female gametogenesis, delving into more detail in these topics.
Male gametogenesis
General hormonal regulation
Spermatozoa (sperm cells) are produced from spermatogonial stem cells (SSCs) within seminiferous tubules in the testis. This occurs under the control of androgens and Follicle Stimulating Hormone (FSH). FSH has a unique chain of 111 amino acids and 6 carbohydrate groups. Androgens are produced outside the tubules by Leydig cells under control of Luteinising Hormone (LH), which also has a unique chain, but of 122 amino acids, and 2 carbohydrate groups. FSH gets released from the anterior (ant.) pituitary to regulate Sertoli cells for spermatogenesis (which will be discussed in more detail later). LH is also released from the ant. Pituitary to Leydig cells, producing androgens; regulating Sertoli cells and thus spermatogenesis.
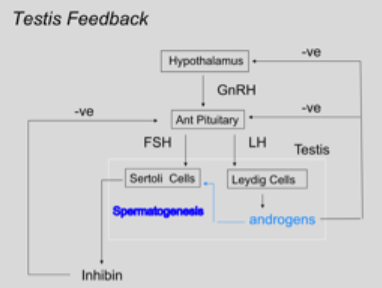
Testicular fluid (where spermatozoa get released in) is produced by Sertoli cells and carries the sperm out of the testis. Inhibin is also produced by these cells under the control of FSH. This causes a negative feedback system to the ant. pituitary, illustrated in the below figure (1).
Figure 1: Negative feedback system for hormonal regulation of male gametogenesis
In the male, only negative feedback occurs: rising testosterone inhibits LH and FSH output and rising inhibin selectively inhibits FSH output. In females, oestrogen exerts both types of feedback: slowly rising oestrogen inhibits LH and FSH output, whilst rapidly rising oestrogen stimulated LH and FSH output.
Spermatogenesis
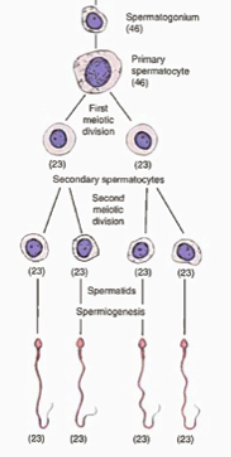
There are three principal elements of spermatogenesis: mitotic proliferation, meiotic division and cytodifferentiation (spermiogenesis). Spermiation is also an important process whereby spermatozoa are released luminally into testicular fluid.
In spermatogenesis, a diploid spermatogonium begins the process by proliferating into the primary spermatocyte, which is also diploid. Mitotic proliferation is hormonally regulated by FSH or testosterone, but that of the formation of primary spermatocyte is unknown. The cell then divides for the first time by meiosis, resulting in two haploid secondary spermatocytes. This is regulated by testosterone. These then undergo a second meiotic division, producing four haploid spermatids, potentially also being regulated by testosterone, but this is not conclusive. which undergo spermiogenesis to produce four motile, fully functioning haploid sperm cells. Spermiogenesis is regulated by FSH, and spermiation by LH. The process of spermatogenesis is depicted in figure 2.
Figure 2: A schematic of spermatogenesis
There is unfortunately little data available for the human male, with the above being based on rat models. Oestradiol from Sertoli cells may also play a role in the regulation of male gametogenesis. Following hypophysectomy, the process arrests in early meiosis and nothing more mature than a primary spermatocyte is seen. The lumen collapses as there is no fluid secretion from Sertoli cells.
Female gametogenesis
Primordial to primary follicle phases
Gametogenesis in females (also known as oogenesis) occurs in the ovaries and begins during foetal development when primordial germ cells migrate to the developing ovaries. These develop into oogonia, the precursor cells of oocytes. Each oogonium replicates its DNA through mitosis and undergoes multiple rounds of cell division, forming a cluster of cells caused primordial follicles. Each primordial follicle consists of an oocyte surrounded by flattened follicular cells.
Within each primordial follicle, one oocyte becomes the primary oocyte, while the surrounding follicular cells form the follicular epithelium layer. Primary oocytes are arrested in prophase I of meiosis, meaning they are in a state of suspended development until puberty. The oocyte begins a major growth phase up until the preantral phase (grows from 10 to 120 in diameter), with lots of RNA and protein synthesis occurring. There is no reactivation of meiosis from the dictyate stage and these stages do not depend on exogenous hormones, nor does it produce them.
Upon reaching puberty, a pool of primordial follicles is activated in each menstrual cycle. During each cycle, under the influence of FSH, a few primordial follicles begin to mature. Typically, only one follicle becomes dominant and continues to develop, while the others undergo atresia (degeneration). The dominant follicle continues to grow and matures, and the primary oocyte within it completes the first meiotic divison, resulting in the formation of a secondary oocyte and a polar body. This secondary oocyte is arrested in metaphase II of meiosis until fertilisation occurs.
Pre-antral to antral stage: antral follicle formation
Gonadotrophins are then required for the formation of the antral follicle, and these are secreted by the ant. pituitary. In structure, these are glycoproteins with 2 peptide chains. They share a common chain, of 92 amino acids and 2 carbohydrate groups.
Sex steroids are required for this transition. Granulosa cells develop FSH receptors and require FSH. Under FSH stimulation, granulosa cell convert androgens to oestrogens via an enzyme called aromatase. Granulosa cells are the female equivalent of Sertoli cells, which also bind FSH. They also produce inhibition for feedback, as well as cAMP and hypoxanthine production. There is a positive feedback mechanism: oestrogens also bind and stimulate the granulosa cells that made them (initial low and then rising level of oestrogen will bring about ovulation). Granulosa cells also use aromatase to convert testosterone to oestrogen and oestradiol.
Analogous to granulosa cells developing FSH receptors and requiring FSH, thecal cells develop LH receptors and require LH. Under LH stimulation, thecal cells are caused to make androgens. In the male, this is analogous to the Leydig cells of the testes (also binding LH). Some of these androgens pass to granulosa cells, which in the presence of FSH, gets converted to oestrogens (called aromatisation). Oestrogens also bind and stimulate the granulosa cells that made them (aforementioned positive feedback system).
Inhibin is made in granulosa cells in the female, whereas in the male, Sertoli cells make it. Inhibin production is low during the preantral phases but starts to rise as the antrum develops. Rising inhibin levels stimulate androgen output by the thecal cells and its conversion to oestrogens by granulosa cells. Thus inhibin also increases the output of oestrogens: in two ways – via positive feedback on granulosa cells, as well as inhibin itself also stimulating more androgens from thecal cells directly – the resulting oestrogen increase being needed for ovulation.
Preovulatory follicle
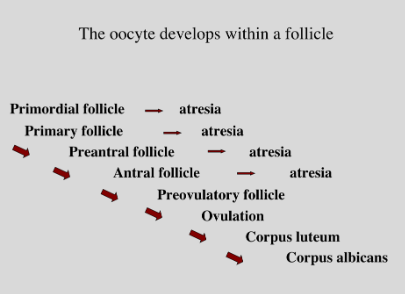
Whilst all these changes to the follicle occur, oocyte meiosis reactivates due to high levels of LH. This causes the first and second meiotic division, including the formation of polar bodies. This eventually arrests a second time at the second meiotic metaphase. The oocyte that gets ovulated is arrested in this stage. This gametogenesis process in females in summarised in the figure below.
Figure 3: Summary of female gametogenesis stages, with atresia occurring in tandem upuntil the preovulatory stage. Follicular atresia is the loss of developing follicles throughapoptotic death of granulosa cells.
Conclusion
In summary, male and female gametogenesis are both complex, hormonally regulated processes, which may be cross compared in multiple aspects. In males, a negative feedback system involving inhibin, FSH and LH dominates androgen production, affecting Leydig and Sertoli cells, affecting spermatogenesis. This process occurs extensively, to produce 3 million sperm cells in the adult male per day. In females, however, the 1-2 oocytes made each month develop within a follicle, which undergoes several stages from the primordial follicle to the ovulated oocyte, all hormonally regulated. Thecal and granulosa cells in the female are essential in the hormonal regulation of gametogenesis by FSH and LH and are analogous in the male to Leydig and Sertoli cells, respectively. Interestingly, it appears that less is known about the hormonal regulation of male than female gametogenesis.
See More Posts
Copyright © 2021 Govest, Inc. All rights reserved.